High-resolution intravascular MRI-guided perivascular ultrasound ablation
- PMID: 31402512
- PMCID: PMC6778713
- DOI: 10.1002/mrm.27932
High-resolution intravascular MRI-guided perivascular ultrasound ablation
Abstract
Purpose: To develop and test in animal studies ex vivo and in vivo, an intravascular (IV) MRI-guided high-intensity focused ultrasound (HIFU) ablation method for targeting perivascular pathology with minimal injury to the vessel wall.
Methods: IV-MRI antennas were combined with 2- to 4-mm diameter water-cooled IV-ultrasound ablation catheters for IV-MRI on a 3T clinical MRI scanner. A software interface was developed for monitoring thermal dose with real-time MRI thermometry, and an MRI-guided ablation protocol developed by repeat testing on muscle and liver tissue ex vivo. MRI thermal dose was measured as cumulative equivalent minutes at 43°C (CEM43 ). The IV-MRI IV-HIFU protocol was then tested by targeting perivascular ablations from the inferior vena cava of 2 pigs in vivo. Thermal dose and lesions were compared by gross and histological examination.
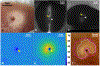
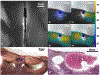
Results: Ex vivo experiments yielded a 6-min ablation protocol with the IV-ultrasound catheter coolant at 3-4°C, a 30 mL/min flow rate, and 7 W ablation power. In 8 experiments, 5- to 10-mm thick thermal lesions of area 0.5-2 cm2 were produced that spared 1- to 2-mm margins of tissue abutting the catheters. The radial depths, areas, and preserved margins of ablation lesions measured from gross histology were highly correlated (r ≥ 0.79) with those measured from the CEM43 = 340 necrosis threshold determined by MRI thermometry. The psoas muscle was successfully targeted in the 2 live pigs, with the resulting ablations controlled under IV-MRI guidance.
Conclusion: IV-MRI-guided, IV-HIFU has potential as a precision treatment option that could preserve critical blood vessel wall during ablation of nonresectable perivascular tumors or other pathologies.
Keywords: MR-guided ultrasound ablation; high intensity focused ultrasound (HIFU); intravascular MRI (IVMRI); liver and pancreatic cancer; vessel involvement.
© 2019 International Society for Magnetic Resonance in Medicine.
Figures







Similar articles
-
Endoluminal ultrasound applicators for MR-guided thermal ablation of pancreatic tumors: Preliminary design and evaluation in a porcine pancreas model.Med Phys. 2016 Jul;43(7):4184. doi: 10.1118/1.4953632. Med Phys. 2016. PMID: 27370138 Free PMC article.
-
A clinically feasible treatment protocol for magnetic resonance-guided high-intensity focused ultrasound ablation in the liver.Invest Radiol. 2015 Jan;50(1):24-31. doi: 10.1097/RLI.0000000000000091. Invest Radiol. 2015. PMID: 25198833
-
Non-Invasive Targeted Peripheral Nerve Ablation Using 3D MR Neurography and MRI-Guided High-Intensity Focused Ultrasound (MR-HIFU): Pilot Study in a Swine Model.PLoS One. 2015 Dec 14;10(12):e0144742. doi: 10.1371/journal.pone.0144742. eCollection 2015. PLoS One. 2015. PMID: 26659073 Free PMC article.
-
Advances in MR image-guided high-intensity focused ultrasound therapy.Int J Hyperthermia. 2015 May;31(3):225-32. doi: 10.3109/02656736.2014.976773. Epub 2014 Nov 6. Int J Hyperthermia. 2015. PMID: 25373687 Review.
-
Principles of focused ultrasound.Minim Invasive Ther Allied Technol. 2018 Feb;27(1):41-50. doi: 10.1080/13645706.2017.1414063. Epub 2018 Jan 18. Minim Invasive Ther Allied Technol. 2018. PMID: 29343145 Review.
Cited by
-
Real-Time High-Resolution MRI Endoscopy at up to 10 Frames per Second.BME Front. 2021 Feb 17;2021:6185616. doi: 10.34133/2021/6185616. eCollection 2021. BME Front. 2021. PMID: 37849906 Free PMC article.
References
-
- Pancreatic Cancer - Cancer Stat Facts. SEER. https://seer.cancer.gov/statfacts/html/pancreas.html. Accessed March 13, 2019.
-
- Petrou A, Moris D, Paul Tabet P, David Wensley Richards B, Kourounis G. Ablation of the locally advanced pancreatic cancer: An introduction and brief summary of techniques. J. BUON Off. J. Balk. Union Oncol. 2016;21:650–658. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

