A single-center SCN8A-related epilepsy cohort: clinical, genetic, and physiologic characterization
- PMID: 31402610
- PMCID: PMC6689675
- DOI: 10.1002/acn3.50839
A single-center SCN8A-related epilepsy cohort: clinical, genetic, and physiologic characterization
Abstract
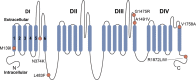
Objective: Pathogenic variants in SCN8A, encoding the voltage-gated sodium (Na+) channel α subunit Nav1.6, is a known cause of epilepsy. Here, we describe clinical and genetic features of all patients with SCN8A epilepsy evaluated at a single-tertiary care center, with biophysical data on identified Nav1.6 variants and pharmacological response to selected Na+ channel blockers.
Methods: SCN8A variants were identified via an exome-based panel of epilepsy-associated genes for next generation sequencing (NGS), or via exome sequencing. Biophysical characterization was performed using voltage-clamp recordings of ionic currents in heterologous cells.
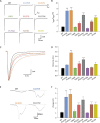
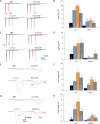
Results: We observed a range in age of onset and severity of epilepsy and associated developmental delay/intellectual disability. Na+ channel blockers were highly or partially effective in most patients. Nav1.6 variants exhibited one or more biophysical defects largely consistent with gain of channel function. We found that clinical severity was correlated with the presence of multiple observed biophysical defects and the extent to which pathological Na+ channel activity could be normalized pharmacologically. For variants not previously reported, functional studies enhanced the evidence of pathogenicity.
Interpretation: We present a comprehensive single-center dataset for SCN8A epilepsy that includes clinical, genetic, electrophysiologic, and pharmacologic data. We confirm a spectrum of severity and a variety of biophysical defects of Nav1.6 variants consistent with gain of channel function. Na+ channel blockers in the treatment of SCN8A epilepsy may correlate with the effect of such agents on pathological Na+ current observed in heterologous systems.
© 2019 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
Conflict of interest statement
None declared.
Figures

Similar articles
-
The novel sodium channel modulator GS-458967 (GS967) is an effective treatment in a mouse model of SCN8A encephalopathy.Epilepsia. 2018 Jun;59(6):1166-1176. doi: 10.1111/epi.14196. Epub 2018 May 21. Epilepsia. 2018. PMID: 29782051 Free PMC article.
-
Genotype-phenotype correlations in SCN8A-related disorders reveal prognostic and therapeutic implications.Brain. 2022 Sep 14;145(9):2991-3009. doi: 10.1093/brain/awab321. Brain. 2022. PMID: 34431999 Free PMC article.
-
Aberrant Sodium Channel Currents and Hyperexcitability of Medial Entorhinal Cortex Neurons in a Mouse Model of SCN8A Encephalopathy.J Neurosci. 2017 Aug 9;37(32):7643-7655. doi: 10.1523/JNEUROSCI.2709-16.2017. Epub 2017 Jul 4. J Neurosci. 2017. PMID: 28676574 Free PMC article.
-
SCN8A Epilepsy, Developmental Encephalopathy, and Related Disorders.Pediatr Neurol. 2021 Sep;122:76-83. doi: 10.1016/j.pediatrneurol.2021.06.011. Epub 2021 Aug 3. Pediatr Neurol. 2021. PMID: 34353676 Review.
-
SCN8A encephalopathy: Mechanisms and models.Epilepsia. 2019 Dec;60 Suppl 3(Suppl 3):S86-S91. doi: 10.1111/epi.14703. Epilepsia. 2019. PMID: 31904118 Free PMC article. Review.
Cited by
-
Autophagy and autophagy signaling in Epilepsy: possible role of autophagy activator.Mol Med. 2023 Oct 25;29(1):142. doi: 10.1186/s10020-023-00742-2. Mol Med. 2023. PMID: 37880579 Free PMC article. Review.
-
Adrenergic Mechanisms of Audiogenic Seizure-Induced Death in a Mouse Model of SCN8A Encephalopathy.Front Neurosci. 2021 Mar 4;15:581048. doi: 10.3389/fnins.2021.581048. eCollection 2021. Front Neurosci. 2021. PMID: 33762902 Free PMC article.
-
Epilepsy-Related Voltage-Gated Sodium Channelopathies: A Review.Front Pharmacol. 2020 Aug 18;11:1276. doi: 10.3389/fphar.2020.01276. eCollection 2020. Front Pharmacol. 2020. PMID: 33013363 Free PMC article. Review.
-
NBI-921352, a first-in-class, NaV1.6 selective, sodium channel inhibitor that prevents seizures in Scn8a gain-of-function mice, and wild-type mice and rats.Elife. 2022 Mar 2;11:e72468. doi: 10.7554/eLife.72468. Elife. 2022. PMID: 35234610 Free PMC article.
-
Rational Small Molecule Treatment for Genetic Epilepsies.Neurotherapeutics. 2021 Jul;18(3):1490-1499. doi: 10.1007/s13311-021-01110-w. Epub 2021 Aug 24. Neurotherapeutics. 2021. PMID: 34431030 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
