A Versatile Biosynthetic Hydrogel Platform for Engineering of Tissue Analogues
- PMID: 31402634
- PMCID: PMC7116179
- DOI: 10.1002/adhm.201900979
A Versatile Biosynthetic Hydrogel Platform for Engineering of Tissue Analogues
Abstract
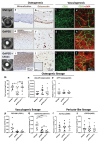
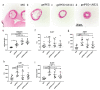
For creating functional tissue analogues in tissue engineering, stem cells require very specific 3D microenvironments to thrive and mature. Demanding (stem) cell types that are used nowadays can find such an environment in a heterogeneous protein mixture with the trade name Matrigel. Several variations of synthetic hydrogel platforms composed of poly(ethylene glycol) (PEG), which are spiked with peptides, have been recently developed and shown equivalence to Matrigel for stem cell differentiation. Here a clinically relevant hydrogel platform, based on PEG and gelatin, which even outperforms Matrigel when targeting 3D prevascularized bone and liver organoid tissue engineering models is presented. The hybrid hydrogel with natural and synthetic components stimulates efficient cell differentiation, superior to Matrigel models. Furthermore, the strength of this hydrogel lies in the option to covalently incorporate unmodified proteins. These results demonstrate how a hybrid hydrogel platform with intermediate biological complexity, when compared to existing biological materials and synthetic PEG-peptide approaches, can efficiently support tissue development from human primary cells.
Keywords: FXIII; Matrigel; gelatin; liver organoids; osteogenesis; polyethylene glycol; vasculogenesis.
© 2019 The Authors. Published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Co-author H.C. has the following competing financial interest to disclose; Scientific co-founder and SAB member of Surrozen (SF); SAB member of Merus, Utrecht; SAB member of Kallyope, NY; SAB member of Decibel, Boston; And the following non-financial interests; Venture partner of LSP, Amsterdam; Inventor on multiple patents related to Lgr5 stem cells and organoids.
Figures



Similar articles
-
The biological macromolecules constructed Matrigel for cultured organoids in biomedical and tissue engineering.Colloids Surf B Biointerfaces. 2025 Mar;247:114435. doi: 10.1016/j.colsurfb.2024.114435. Epub 2024 Dec 6. Colloids Surf B Biointerfaces. 2025. PMID: 39647422 Review.
-
Cytocompatibility testing of hydrogels toward bioprinting of mesenchymal stem cells.J Biomed Mater Res A. 2017 Dec;105(12):3231-3241. doi: 10.1002/jbm.a.36179. Epub 2017 Aug 22. J Biomed Mater Res A. 2017. PMID: 28782179
-
Mechanically and Chemically Defined PEG Hydrogels Improve Reproducibility in Human Cardioid Development.Adv Healthc Mater. 2025 Jun;14(16):e2403997. doi: 10.1002/adhm.202403997. Epub 2025 May 16. Adv Healthc Mater. 2025. PMID: 40376871 Free PMC article.
-
A Genome-wide Analysis of Human Pluripotent Stem Cell-Derived Endothelial Cells in 2D or 3D Culture.Stem Cell Reports. 2017 Apr 11;8(4):907-918. doi: 10.1016/j.stemcr.2017.02.014. Epub 2017 Mar 23. Stem Cell Reports. 2017. PMID: 28343999 Free PMC article.
-
Non-matrigel scaffolds for organoid cultures.Cancer Lett. 2021 Apr 28;504:58-66. doi: 10.1016/j.canlet.2021.01.025. Epub 2021 Feb 11. Cancer Lett. 2021. PMID: 33582211 Review.
Cited by
-
Using Liver Organoids as Models to Study the Pathobiology of Rare Liver Diseases.Biomedicines. 2024 Feb 17;12(2):446. doi: 10.3390/biomedicines12020446. Biomedicines. 2024. PMID: 38398048 Free PMC article. Review.
-
Engineering Dental Tissues Using Biomaterials with Piezoelectric Effect: Current Progress and Future Perspectives.J Funct Biomater. 2022 Dec 22;14(1):8. doi: 10.3390/jfb14010008. J Funct Biomater. 2022. PMID: 36662055 Free PMC article. Review.
-
Addressing present pitfalls in 3D printing for tissue engineering to enhance future potential.APL Bioeng. 2020 Feb 10;4(1):010901. doi: 10.1063/1.5127860. eCollection 2020 Mar. APL Bioeng. 2020. PMID: 32072121 Free PMC article.
-
A novel vascularized urethra-on-a-chip model.Sci Rep. 2025 Mar 7;15(1):8062. doi: 10.1038/s41598-025-92485-9. Sci Rep. 2025. PMID: 40055501 Free PMC article.
-
Organotypic stromal cells impact endothelial cell transcriptome in 3D microvessel networks.Sci Rep. 2022 Nov 28;12(1):20434. doi: 10.1038/s41598-022-24013-y. Sci Rep. 2022. PMID: 36443378 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

