Biology of Infection and Disease Pathogenesis to Guide RSV Vaccine Development
- PMID: 31402910
- PMCID: PMC6677153
- DOI: 10.3389/fimmu.2019.01675
Biology of Infection and Disease Pathogenesis to Guide RSV Vaccine Development
Abstract
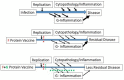
Respiratory syncytial virus (RSV) is a leading cause of severe lower respiratory tract disease in young children and a substantial contributor to respiratory tract disease throughout life and as such a high priority for vaccine development. However, after nearly 60 years of research no vaccine is yet available. The challenges to developing an RSV vaccine include the young age, 2-4 months of age, for the peak of disease, the enhanced RSV disease associated with the first RSV vaccine, formalin-inactivated RSV with an alum adjuvant (FI-RSV), and difficulty achieving protection as illustrated by repeat infections with disease that occur throughout life. Understanding the biology of infection and disease pathogenesis has and will continue to guide vaccine development. In this paper, we review the roles that RSV proteins play in the biology of infection and disease pathogenesis and the corresponding contribution to live attenuated and subunit RSV vaccines. Each of RSV's 11 proteins are in the design of one or more vaccines. The G protein's contribution to disease pathogenesis through altering host immune responses as well as its role in the biology of infection suggest it can make a unique contribution to an RSV vaccine, both live attenuated and subunit vaccines. One of G's potential unique contributions to a vaccine is the potential for anti-G immunity to have an anti-inflammatory effect independent of virus replication. Though an anti-viral effect is essential to an effective RSV vaccine, it is important to remember that the goal of a vaccine is to prevent disease. Thus, other effects of the infection, such as G's alteration of the host immune response may provide opportunities to induce responses that block this effect and improve an RSV vaccine. Keeping in mind the goal of a vaccine is to prevent disease and not virus replication may help identify new strategies for other vaccine challenges, such as improving influenza vaccines and developing HIV vaccines.
Keywords: RSV (respiratory syncytial virus); biology of infection; pathogenesis; protective immunity; vaccine development.
Figures
References
-
- Shi T, McAllister DA, O'Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al. . Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. (2017) 390:946–58. 10.1016/S0140-6736(17)30938-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

