Adaptive and Innate Immunity in Psoriasis and Other Inflammatory Disorders
- PMID: 31402919
- PMCID: PMC6676248
- DOI: 10.3389/fimmu.2019.01764
Adaptive and Innate Immunity in Psoriasis and Other Inflammatory Disorders
Abstract
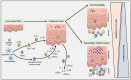
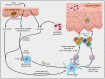
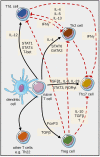
Over the past three decades, a considerable body of evidence has highlighted T cells as pivotal culprits in the pathogenesis of psoriasis. This includes the association of psoriasis with certain MHC (HLA) alleles, oligoclonal expansion of T cells in some cases, therapeutic response to T cell-directed immunomodulation, the onset of psoriasis following bone marrow transplantation, or induction of psoriasis-like inflammation by T cells in experimental animals. There is accumulating clinical and experimental evidence suggesting that both autoimmune and autoinflammatory mechanisms lie at the core of the disease. Indeed, some studies suggested antigenic functions of structural proteins, and complexes of self-DNA with cathelicidin (LL37) or melanocytic ADAMTSL5 have been proposed more recently as actual auto-antigens in some cases of psoriasis. These findings are accompanied by various immunoregulatory mechanisms, which we increasingly understand and which connect innate and adaptive immunity. Specific adaptive autoimmune responses, together with our current view of psoriasis as a systemic inflammatory disorder, raise the question of whether psoriasis may have connections to autoimmune or autoinflammatory disorders elsewhere in the body. While such associations have been suspected for many years, compelling mechanistic evidence in support of this notion is still scant. This review sets into context the current knowledge about innate and adaptive immunological processes in psoriasis and other autoimmune or autoinflammatory diseases.
Keywords: adaptive immunity; autoimmune disease; innate immunity; psoriasis; skin—immunology.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

