Bone remodeling effect of a chitosan and calcium phosphate-based composite
- PMID: 31402983
- PMCID: PMC6683952
- DOI: 10.1093/rb/rbz009
Bone remodeling effect of a chitosan and calcium phosphate-based composite
Abstract
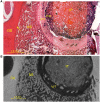
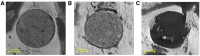
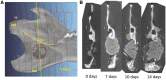
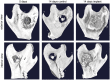
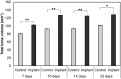
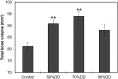
Chitosan is a biocompatible polymer that has been widely studied for tissue engineering purposes. The aim of this research was to assess bone regenerative properties of an injectable chitosan and calcium phosphate-based composite and identify optimal degree of deacetylation (%DDA) of the chitosan polymer. Drill holes were generated on the left side of a mandible in Sprague-Dawley rats, and the hole was either left empty or filled with the implant. The animals were sacrificed at several time points after surgery (7-22 days) and bone was investigated using micro-CT and histology. No significant new bone formation was observed in the implants themselves at any time points. However, substantial new bone formation was observed in the rat mandible further away from the drill hole. Morphological changes indicating bone formation were found in specimens explanted on Day 7 in animals that received implant. Similar bone formation pattern was seen in control animals with an empty drill hole at later time points but not to the same extent. A second experiment was performed to examine if the %DDA of the chitosan polymer influenced the bone remodeling response. The results suggest that chitosan polymers with %DDA between 50 and 70% enhance the natural bone remodeling mechanism.
Keywords: bone defects; bone implant; bone remodeling; chitosan; degree of deacetylation; micro-CT; rat mandible.
Figures


References
-
- Carragee EJ, Hurwitz EL, Weiner BK.. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 2011;11:471–91. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

