Linked Toll-Like Receptor Triagonists Stimulate Distinct, Combination-Dependent Innate Immune Responses
- PMID: 31403067
- PMCID: PMC6661867
- DOI: 10.1021/acscentsci.8b00823
Linked Toll-Like Receptor Triagonists Stimulate Distinct, Combination-Dependent Innate Immune Responses
Abstract
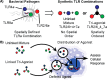
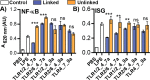
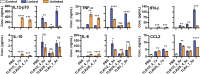
Traditional vaccination strategies have failed to generate effective vaccines for many infections like tuberculosis and HIV. New approaches are needed for each type of disease. The protective immunity and distinct responses of many successful vaccines come from activating multiple Toll-like receptors (TLRs). Vaccines with multiple TLRs as adjuvants have proven effective in preclinical studies, but current research has not explored two important elements. First, few multi-TLR systems explore spatial organization-a critical feature of whole-cell vaccines. Second, no multi-TLR systems to date provide systematic analysis of the combinatorial space of three TLR agonists. Here, we present the first examination of the combinatorial space of several spatially defined triple-TLR adjuvants, by synthesizing a series of five triple-TLR agonists and testing their innate activity both in vitro and in vivo. The combinations were evaluated by measuring activation of immune stimulatory genes (Nf-κB, ISGs), cytokine profiles (IL12-p70, TNF-α, IL-6, IL-10, CCL2, IFN-α, IFN-β, IFN-γ), and in vivo cytokine serum levels (IL-6, TNF-α, IL12-p40, IFN-α, IFN-β). We demonstrate that linking TLR agonists substantially alters the resulting immune response compared to their unlinked counterparts and that each combination results in a distinct immune response, particularly between linked combinations. We show that combinations containing a TLR9 agonist produce more Th1 biasing immune response profiles, and that the effect is amplified upon conjugation. However, combinations containing TLR2/6 agonist are skewed toward TH2 biasing profiles despite the presence of TLR9. These results demonstrate the profound effects that conjugation and combinatorial administration of TLR agonists can have on immune responses, a critical element of vaccine development.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Querec T.; Bennouna S.; Alkan S.; Laouar Y.; Gorden K.; Flavell R.; Akira S.; Ahmed R.; Pulendran B. Yellow fever vaccine yf-17d activates multiple dendritic cell subsets via tlr2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 2006, 203 (2), 413–424. 10.1084/jem.20051720. - DOI - PMC - PubMed
-
- Timmermans K.; Plantinga T. S.; Kox M.; Vaneker M.; Scheffer G. J.; Adema G. J.; Joosten L. A. B.; Netea M. G. Blueprints of signaling interactions between pattern recognition receptors: implications for the design of vaccine adjuvants. Clin. Vaccine Immunol. 2013, 20 (3), 427–432. 10.1128/CVI.00703-12. - DOI - PMC - PubMed

