Biowire Model of Interstitial and Focal Cardiac Fibrosis
- PMID: 31403068
- PMCID: PMC6661857
- DOI: 10.1021/acscentsci.9b00052
Biowire Model of Interstitial and Focal Cardiac Fibrosis
Abstract
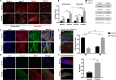
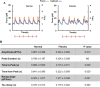
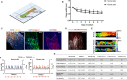
Myocardial fibrosis is a severe global health problem due to its prevalence in all forms of cardiac diseases and direct role in causing heart failure. The discovery of efficient antifibrotic compounds has been hampered due to the lack of a physiologically relevant disease model. Herein, we present a disease model of human myocardial fibrosis and use it to establish a compound screening system. In the Biowire II platform, cardiac tissues are suspended between a pair of poly(octamethylene maleate (anhydride) citrate) (POMaC) wires. Noninvasive functional readouts are realized on the basis of the deflection of the intrinsically fluorescent polymer. The disease model is constructed to recapitulate contractile, biomechanical, and electrophysiological complexities of fibrotic myocardium. Additionally, we constructed a heteropolar integrated model with fibrotic and healthy cardiac tissues coupled together. The integrated model captures the regional heterogeneity of scar lesion, border zone, and adjacent healthy myocardium. Finally, we demonstrate the utility of the system for the evaluation of antifibrotic compounds. The high-fidelity in vitro model system combined with convenient functional readouts could potentially facilitate the development of precision medicine strategies for cardiac fibrosis modeling and establish a pipeline for preclinical compound screening.
Conflict of interest statement
The authors declare the following competing financial interest(s): Y.Z. and M.R. are co-founders of TARA Biosystems Inc. and hold equity in this company. TARA Biosystems Inc. uses the Biowire II technology described in this manuscript for commercial applications. All other authors have no conflicts of interest.
Figures



Similar articles
-
Design and Fabrication of Biological Wires for Cardiac Fibrosis Disease Modeling.Methods Mol Biol. 2022;2485:175-190. doi: 10.1007/978-1-0716-2261-2_12. Methods Mol Biol. 2022. PMID: 35618906
-
3D Printability Assessment of Poly(octamethylene maleate (anhydride) citrate) and Poly(ethylene glycol) Diacrylate Copolymers for Biomedical Applications.ACS Appl Polym Mater. 2022 Aug 12;4(8):5457-5470. doi: 10.1021/acsapm.2c00531. Epub 2022 Jul 7. ACS Appl Polym Mater. 2022. PMID: 35991303 Free PMC article.
-
Oncofetal Protein CRIPTO Is Involved in Wound Healing and Fibrogenesis in the Regenerating Liver and Is Associated with the Initial Stages of Cardiac Fibrosis.Cells. 2021 Nov 26;10(12):3325. doi: 10.3390/cells10123325. Cells. 2021. PMID: 34943832 Free PMC article.
-
Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration.Cell Tissue Res. 2016 Sep;365(3):563-81. doi: 10.1007/s00441-016-2431-9. Epub 2016 Jun 21. Cell Tissue Res. 2016. PMID: 27324127 Free PMC article. Review.
-
Myocardial interstitial fibrosis in the era of precision medicine. Biomarker-based phenotyping for a personalized treatment.Rev Esp Cardiol (Engl Ed). 2020 Mar;73(3):248-254. doi: 10.1016/j.rec.2019.09.010. Epub 2019 Nov 20. Rev Esp Cardiol (Engl Ed). 2020. PMID: 31759935 Review. English, Spanish.
Cited by
-
Tissue-in-a-Tube: three-dimensional in vitro tissue constructs with integrated multimodal environmental stimulation.Mater Today Bio. 2020 Jul 28;7:100070. doi: 10.1016/j.mtbio.2020.100070. eCollection 2020 Jun. Mater Today Bio. 2020. PMID: 32875285 Free PMC article.
-
The new era of cardiovascular research: revolutionizing cardiovascular research with 3D models in a dish.Med Rev (2021). 2024 Feb 20;4(1):68-85. doi: 10.1515/mr-2023-0059. eCollection 2024 Feb. Med Rev (2021). 2024. PMID: 38515776 Free PMC article. Review.
-
Flexible 3D printed microwires and 3D microelectrodes for heart-on-a-chip engineering.Biofabrication. 2023 Jun 22;15(3):035023. doi: 10.1088/1758-5090/acd8f4. Biofabrication. 2023. PMID: 37230083 Free PMC article.
-
Action potential metrics and automated data analysis pipeline for cardiotoxicity testing using optically mapped hiPSC-derived 3D cardiac microtissues.PLoS One. 2023 Feb 6;18(2):e0280406. doi: 10.1371/journal.pone.0280406. eCollection 2023. PLoS One. 2023. PMID: 36745602 Free PMC article.
-
Disease-inspired tissue engineering: Investigation of cardiovascular pathologies.ACS Biomater Sci Eng. 2020 May 11;6(5):2518-2532. doi: 10.1021/acsbiomaterials.9b01067. Epub 2019 Oct 29. ACS Biomater Sci Eng. 2020. PMID: 32974421 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

