Fine-Tuning the Pore Environment of the Microporous Cu-MOF for High Propylene Storage and Efficient Separation of Light Hydrocarbons
- PMID: 31403074
- PMCID: PMC6661871
- DOI: 10.1021/acscentsci.9b00423
Fine-Tuning the Pore Environment of the Microporous Cu-MOF for High Propylene Storage and Efficient Separation of Light Hydrocarbons
Abstract
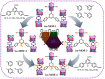
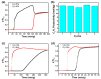
Ethylene (C2H4) and propylene (C3H6) are important energy sources and raw materials in the chemical industry. Storage and separation of C2H4 and C3H6 are vital to their practical application. Metal-organic frameworks (MOFs) having adjustable structures and pore environments are promising candidates for C3H6/C2H4 separation. Herein, we obtained a Cu-based MOF synthesized by H3TTCA and pyrazine ligands. By adding different functional groups on the ligands within the MOFs, their pore environments are adjusted, and thus, the C3H6 storage capacity and C3H6/C2H4 separation efficiency are improved. Eventually, the fluoro- and methyl-functionalized iso-MOF-4 exhibits a better gas storage and C3H6/C2H4 separation performance compared with iso-MOF-1 (nonfunctionalized), iso-MOF-2 (fluoro-functionalized), and iso-MOF-3 (methyl-functionalized). A record-high C3H6 uptake of 293.6 ± 2.3 cm3 g-1 (273 K, 1 atm) is achieved using iso-MOF-4. Moreover, iso-MOF-4 shows excellent repeatability, and only 3.5% of C3H6 storage capacities decrease after nine cycles. Employing Grand Canonical Monte Carlo (GCMC) simulations, it is indicated that iso-MOF-4 preferentially adsorbs C3H6 rather than C2H4 at low pressure. Single-crystal X-ray diffraction on C3H6-adsorbed iso-MOF-4 crystals precisely demonstrates the adsorption positions and arrangement of C3H6 molecules in the framework, which is consistent with the theoretical simulations. Remarkably, gas sorption isotherms, molecular simulations, and breakthrough experiments comprehensively demonstrate that this unique MOF material exhibits highly efficient C3H6/C2H4 separation. Additionally, iso-MOF-4 also possesses efficient separation of C3H8/CH4 and C2H6/CH4, indicating its promising potential in storage/separation of light hydrocarbons in industry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Hao H. G.; Zhao Y. F.; Chen D. M.; Yu J. M.; Tan K.; Ma S. Q.; Chabal Y.; Zhang Z. M.; Dou J. M.; Xiao Z. H.; Day G.; Zhou H. C.; Lu T. B. Simultaneous trapping of C2H2 and C2H6 from a ternary mixture of C2H2/C2H4/C2H6 in a robust metal-organic framework for the purification of C2H4. Angew. Chem., Int. Ed. 2018, 57 (49), 16067–16071. 10.1002/anie.201809884. - DOI - PubMed
-
- Wang H.; Dong X. L.; Colombo V.; Wang Q. N.; Liu Y. Y.; Liu W.; Wang X. L.; Huang X. Y.; Proserpio D. M.; Sironi A.; Han Y.; Li J. Tailor-made microporous metal-organic frameworks for the full separation of propane from propylene through selective size exclusion. Adv. Mater. 2018, 30, 1805088. 10.1002/adma.201805088. - DOI - PubMed
-
- Liu X.; Ren S.; Zeng G. F.; Liu G. J.; Wu P.; Wang G.; Chen X. Q.; Liu Z. Y.; Sun Y. H. Coke suppression in MTO over hierarchical SAPO-34 zeolites. RSC Adv. 2016, 6 (34), 28787–28791. 10.1039/C6RA02282K. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

