Tanshinones: First-in-Class Inhibitors of the Biogenesis of the Type 3 Secretion System Needle of Pseudomonas aeruginosa for Antibiotic Therapy
- PMID: 31403076
- PMCID: PMC6662154
- DOI: 10.1021/acscentsci.9b00452
Tanshinones: First-in-Class Inhibitors of the Biogenesis of the Type 3 Secretion System Needle of Pseudomonas aeruginosa for Antibiotic Therapy
Abstract
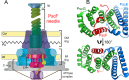
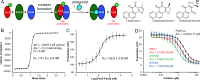
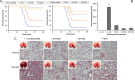
The type 3 secretion system (T3SS) found as cell-surface appendages of many pathogenic Gram-negative bacteria, although nonessential for bacterial survival, is an important therapeutic target for drug discovery and development aimed at inhibiting bacterial virulence without inducing antibiotic resistance. We designed a fluorescence-polarization-based assay for high-throughput screening as a mechanistically well-defined general strategy for antibiotic discovery targeting the T3SS and made a serendipitous discovery of a subset of tanshinones-natural herbal compounds in traditional Chinese medicine widely used for the treatment of cardiovascular and cerebrovascular diseases-as effective inhibitors of the biogenesis of the T3SS needle of multi-drug-resistant Pseudomonas aeruginosa. By inhibiting the T3SS needle assembly and, thus, cytotoxicity and pathogenicity, selected tanshinones reduced the secretion of bacterial virulence factors toxic to macrophages in vitro, and rescued experimental animals challenged with lethal doses of Pseudomonas aeruginosa in a murine model of acute pneumonia. As first-in-class inhibitors with a demonstrable safety profile in humans, tanshinones may be used directly to alleviate Pseudomonas-aeruginosa-associated pulmonary infections without inducing antibiotic resistance. Since the T3SS is highly conserved among Gram-negative bacteria, this antivirulence strategy may be applicable to the discovery and development of novel classes of antibiotics refractory to existing resistance mechanisms for the treatment of many bacterial infections.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- O’Neill J.Review on antimicobial resistance. In Antimicrobial reisistance: tackling a crisis for the health and wealth of nations; The UK Government and the Wellcome Trust, 2014.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

