New family with HSPB 8-associated autosomal dominant rimmed vacuolar myopathy
- PMID: 31403083
- PMCID: PMC6659134
- DOI: 10.1212/NXG.0000000000000349
New family with HSPB 8-associated autosomal dominant rimmed vacuolar myopathy
Abstract
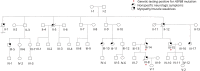
Objective: We clinically and molecularly characterize a new family with autosomal dominant rimmed vacuolar myopathy (RVM) caused by mutations in the HSPB8 gene.
Methods: We performed whole-exome and whole-genome sequencing in the family. Western blot and immunocytochemistry were used to analyze 3 patient fibroblasts, and findings were compared with their age- and sex-matched controls.
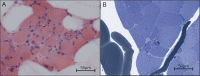
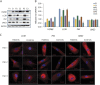
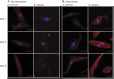
Results: Affected patients have distal and proximal myopathy, with muscle biopsy showing rimmed vacuoles, muscle fiber atrophy, and endomysial fibrosis typical of RVM. Muscle MRI showed severe relatively symmetric multifocal fatty degenerative changes of the lower extremities. We identified a duplication of C at position 515 of the HSPB8 gene (c.515dupC) by whole-genome sequencing, which caused a frameshift with a predicted alternate stop codon p.P173SFS*43 in all affected individuals, resulting in an elongated protein product. Western blot and immunocytochemistry studies revealed reduced expression of heat shock protein beta 8 in patient fibroblasts compared with control fibroblasts, in addition to disrupted autophagy pathology.
Conclusions: We report a novel family with autosomal dominant RVM caused by the c.515dupC mutation of the HSPB8 gene, causing a translational frameshift that results in an elongated protein. Understanding the mechanism for the RVM pathology caused by mutated chaperone will permit novel targeted strategies to alter the natural history progression. As next-generation sequencing becomes more available, additional myopathic families will be identified with HSPB8 mutations.
Figures


References
-
- Arndt V, Dick N, Tawo R, et al. Chaperone-assisted selective autophagy is essential for muscle maintenance. Curr Biol 2010;20:143–148. - PubMed
-
- Fujita M, Mitsuhashi H, Isogai S, et al. Filamin C plays an essential role in the maintenance of the structural integrity of cardiac and skeletal muscles, revealed by the medaka mutant zacro. Dev Biol 2012;361:79–89. - PubMed
