Gain control in the sensorimotor system
- PMID: 31403088
- PMCID: PMC6688851
- DOI: 10.1016/j.cophys.2019.03.005
Gain control in the sensorimotor system
Abstract
Coordinated movement depends on constant interaction between neural circuits that produce motor output and those that report sensory consequences. Fundamental to this process are mechanisms for controlling the influence that sensory signals have on motor pathways - for example, reducing feedback gains when they are disruptive and increasing gains when advantageous. Sensory gain control comes in many forms and serves diverse purposes - in some cases sensory input is attenuated to maintain movement stability and filter out irrelevant or self-generated signals, or enhanced to facilitate salient signals for improved movement execution and adaptation. The ubiquitous presence of sensory gain control across species at multiple levels of the nervous system reflects the importance of tuning the impact that feedback information has on behavioral output.
Keywords: gain control; motor corrections; motor output; movement stability; reafference; sensorimotor adaptation; sensory feedback.
Conflict of interest statement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Figures
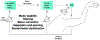

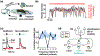
Similar articles
-
Rapid visuomotor feedback gains are tuned to the task dynamics.J Neurophysiol. 2017 Nov 1;118(5):2711-2726. doi: 10.1152/jn.00748.2016. Epub 2017 Aug 23. J Neurophysiol. 2017. PMID: 28835530 Free PMC article.
-
Sensorimotor integration in the whisker somatosensory brain stem trigeminal loop.J Neurophysiol. 2019 Nov 1;122(5):2061-2075. doi: 10.1152/jn.00116.2019. Epub 2019 Sep 18. J Neurophysiol. 2019. PMID: 31533013
-
Sensory prediction errors drive cerebellum-dependent adaptation of reaching.J Neurophysiol. 2007 Jul;98(1):54-62. doi: 10.1152/jn.00266.2007. Epub 2007 May 16. J Neurophysiol. 2007. PMID: 17507504
-
Learning in sensorimotor circuits.Curr Opin Neurobiol. 2004 Dec;14(6):693-7. doi: 10.1016/j.conb.2004.10.009. Curr Opin Neurobiol. 2004. PMID: 15582370 Review.
-
Advantages of comparative studies in songbirds to understand the neural basis of sensorimotor integration.J Neurophysiol. 2017 Aug 1;118(2):800-816. doi: 10.1152/jn.00623.2016. Epub 2017 Mar 22. J Neurophysiol. 2017. PMID: 28331007 Free PMC article. Review.
Cited by
-
Sensory Coding of Limb Kinematics in Motor Cortex across a Key Developmental Transition.J Neurosci. 2021 Aug 11;41(32):6905-6918. doi: 10.1523/JNEUROSCI.0921-21.2021. Epub 2021 Jul 19. J Neurosci. 2021. PMID: 34281990 Free PMC article.
-
Mechanosensory Control of Locomotion in Animals and Robots: Moving Forward.Integr Comp Biol. 2023 Aug 23;63(2):450-463. doi: 10.1093/icb/icad057. Integr Comp Biol. 2023. PMID: 37279901 Free PMC article. Review.
-
The influence of afferent input on somatosensory suppression during grasping.Sci Rep. 2020 Oct 29;10(1):18692. doi: 10.1038/s41598-020-75610-8. Sci Rep. 2020. PMID: 33122705 Free PMC article.
-
Effectiveness and safety of subthreshold vibration over suprathreshold vibration in treatment of muscle fatigue in elderly people.World J Clin Cases. 2023 May 26;11(15):3434-3443. doi: 10.12998/wjcc.v11.i15.3434. World J Clin Cases. 2023. PMID: 37383890 Free PMC article. Review.
-
Future spinal reflex is embedded in primary motor cortex output.Sci Adv. 2024 Dec 20;10(51):eadq4194. doi: 10.1126/sciadv.adq4194. Epub 2024 Dec 18. Sci Adv. 2024. PMID: 39693430 Free PMC article.
References
-
- Cole J: Pride and a daily marathon edn 1st MIT Press Cambridge, Mass: MIT Press; 1995.
-
- Scott SH, Cluff T, Lowrey CR, Takei T: Feedback control during voluntary motor actions. Curr Opin Neurobiol 2015, 33:85–94. - PubMed
-
- Scott SH: A Functional Taxonomy of Bottom-Up Sensory Feedback Processing for Motor Actions. Trends Neurosci 2016, 39:512–526. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
