Human Papillomavirus Genotyping Compared With a Qualitative High-Risk Human Papillomavirus Test After Treatment of High-Grade Cervical Intraepithelial Neoplasia: A Systematic Review
- PMID: 31403602
- PMCID: PMC6727902
- DOI: 10.1097/AOG.0000000000003409
Human Papillomavirus Genotyping Compared With a Qualitative High-Risk Human Papillomavirus Test After Treatment of High-Grade Cervical Intraepithelial Neoplasia: A Systematic Review
Abstract
Objective: To systematically examine human papillomavirus (HPV) genotyping compared with qualitative high-risk HPV result during follow-up after treatment of high-grade cervical intraepithelial neoplasia (CIN), for risk estimation of posttreatment high-grade CIN.
Data sources: MEDLINE, Cochrane, and ClinicalTrials.gov were searched from January 2000 to April 2019 for prospective studies of women and retrospective studies of residual specimens from women, tested using HPV assays with genotype reporting.
Methods of study selection: The primary outcome was posttreatment high-grade CIN after treatment of high-grade CIN. Risk of bias (individual study quality) was evaluated with a modified Newcastle-Ottawa Scale. Overall quality of evidence for the risk estimate outcomes was evaluated using modified GRADE methodology for observational diagnostic studies.
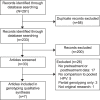
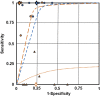
Tabulation, integration, and results: Of the 233 identified abstracts, 33 full-text articles were retrieved, and seven studies were included in the synthesis. The risk of bias was deemed to be low. Either a positive qualitative HPV test result or a positive test result for the same genotype that was present pretreatment have a sensitivity for predicting posttreatment high-grade CIN that approaches 100%. However, the positive predictive value (PPV) for the same genotype result pretreatment and posttreatment (median 44.4%) is about double the PPV (median 22.2%) for qualitative HPV results. The PPV of a new HPV infection posttreatment approximates zero. Human papillomavirus genotyping discriminated risk of posttreatment high-grade CIN to a clinically significant degree for women after treatment procedures for high-grade CIN lesions, when same-genotype persistence was compared with new genotype infection.
Conclusion: There is moderately high-quality evidence to support the improved clinical utility of HPV genotyping compared with qualitative HPV positivity to follow-up after treatment of high-grade CIN.
Systematic review registration: PROSPERO: CRD42018091095.
Funding source: Becton, Dickinson and Company, BD Life Sciences-Diagnostic Systems.
Figures
Comment in
-
Precision Screening for Posttreatment Surveillance.Obstet Gynecol. 2019 Sep;134(3):450-451. doi: 10.1097/AOG.0000000000003438. Obstet Gynecol. 2019. PMID: 31403581 No abstract available.
References
-
- Cuschieri K, Bhatia R, Cruickshank M, Hillemanns P, Arbyn M. HPV testing in the context of post-treatment follow up (test of cure). J Clin Virol 2016;76(suppl 1):S56–61. - PubMed
-
- Arbyn M, Paraskevaidis E, Martin-Hirsch P, Prendiville W, Dillner J. Clinical utility of HPV-DNA detection: triage of minor cervical lesions, follow-up of women treated for high-grade CIN: an update of pooled evidence. Gynecol Oncol 2005;99(3 suppl 1):S7–11. - PubMed
-
- Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 2012;30(suppl 5):F88–99. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

