Use of an Influenza Antigen Microarray to Measure the Breadth of Serum Antibodies Across Virus Subtypes
- PMID: 31403629
- PMCID: PMC11177630
- DOI: 10.3791/59973
Use of an Influenza Antigen Microarray to Measure the Breadth of Serum Antibodies Across Virus Subtypes
Abstract
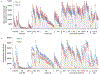
The influenza virus remains a significant cause of mortality worldwide due to the limited effectiveness of currently available vaccines. A key challenge to the development of universal influenza vaccines is high antigenic diversity resulting from antigenic drift. Overcoming this challenge requires novel research tools to measure the breadth of serum antibodies directed against many virus strains across different antigenic subtypes. Here, we present a protocol for analyzing the breadth of serum antibodies against diverse influenza virus strains using a protein microarray of influenza antigens. This influenza antigen microarray is constructed by printing purified hemagglutinin and neuraminidase antigens onto a nitrocellulose-coated membrane using a microarray printer. Human sera are incubated on the microarray to bind antibodies against the influenza antigens. Quantum-dot-conjugated secondary antibodies are used to simultaneously detect IgG and IgA antibodies binding to each antigen on the microarray. Quantitative antibody binding is measured as fluorescence intensity using a portable imager. Representative results are shown to demonstrate assay reproducibility in measuring subtype-specific and cross-reactive influenza antibodies in human sera. Compared to traditional methods such as ELISA, the influenza antigen microarray provides a high throughput multiplexed approach capable of testing hundreds of sera for multiple antibody isotypes against hundreds of antigens in a short time frame, and thus has applications in sero-surveillance and vaccine development. A limitation is the inability to distinguish binding antibodies from neutralizing antibodies.
Figures




References
-
- Murray CJ et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 380 (9859), 2197–2223 (2012). - PubMed
-
- Renegar KB, Crouse D, Floyd RA, Krueger J. Progression of influenza viral infection through the murine respiratory tract: the protective role of sleep deprivation. Sleep. 23 (7), 859–863 (2000). - PubMed
-
- Fauci AS Seasonal and pandemic influenza preparedness: science and countermeasures. Journal of Infectious Diseases. 194 Suppl 2, S73–76 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
