Impact of manganese on biofilm formation and cell morphology of Candida parapsilosis clinical isolates with different biofilm forming abilities
- PMID: 31403663
- PMCID: PMC6761954
- DOI: 10.1093/femsyr/foz057
Impact of manganese on biofilm formation and cell morphology of Candida parapsilosis clinical isolates with different biofilm forming abilities
Abstract
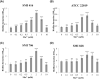
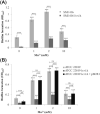
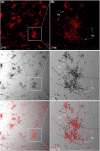
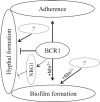
The commensal species Candida parapsilosis is an emerging human pathogen that has the ability to form biofilms. In this study, we explored the impact of the divalent cations cobalt (Co2+), copper (Cu2+), iron (Fe3+), manganese (Mn2+), nickel (Ni2+) and zinc (Zn2+) on biofilm formation of clinical isolates of C. parapsilosis with no, low and high biofilm forming abilities at 30 and 37°C. All strains besides one isolate showed a concentration-dependent enhancement of biofilm formation at 30°C in the presence of Mn2+ with a maximum at 2 mM. The biofilm forming ability of no and low biofilm forming isolates was >2-fold enhanced in the presence of 2 mM Mn2+, while the effect in high biofilm forming isolate was significantly less pronounced. Of note, cells in the biofilms of no and low biofilm forming strains differentiated into yeast and pseudohyphal cells similar in morphology to high biofilm formers. The biofilm transcriptional activator BCR1 has a dual developmental role in the absence and presence of 2 mM Mn2+ as it promoted biofilm formation of no biofilm forming strains, and, surprisingly, suppressed cells of no biofilm forming strains to develop into pseudohyphae and/or hyphae. Thus, environmental conditions can significantly affect the amount of biofilm formation and cell morphology of C. parapsilosis with Mn2+ to overcome developmental blocks to trigger biofilm formation and to partially relieve BCR1 suppressed cell differentiation.
Keywords: BCR1; Candida parapsilosis; biofilm formation; cell morphology; hyphae; manganese; metal ions; pseudohyphae; yeast cells.
© FEMS 2019.
Figures





References
-
- Araújo D, Henriques M, Silva S. Portrait of Candida species biofilm regulatory network genes. Trends Microbiol. 2017;25:62–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

