Neurite orientation dispersion and density imaging (NODDI) and free-water imaging in Parkinsonism
- PMID: 31403737
- PMCID: PMC6865390
- DOI: 10.1002/hbm.24760
Neurite orientation dispersion and density imaging (NODDI) and free-water imaging in Parkinsonism
Abstract
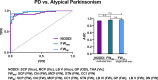
Neurite orientation dispersion and density imaging (NODDI) uses a three-compartment model to probe brain tissue microstructure, whereas free-water (FW) imaging models two-compartments. It is unknown if NODDI detects more disease-specific effects related to neurodegeneration in Parkinson's disease (PD) and atypical Parkinsonism. We acquired multi- and single-shell diffusion imaging at 3 Tesla across two sites. NODDI (using multi-shell; isotropic volume [Viso]; intracellular volume [Vic]; orientation dispersion [ODI]) and FW imaging (using single-shell; FW; free-water corrected fractional anisotropy [FAt]) were compared with 44 PD, 21 multiple system atrophy Parkinsonian variant (MSAp), 26 progressive supranuclear palsy (PSP), and 24 healthy control subjects in the basal ganglia, midbrain/thalamus, cerebellum, and corpus callosum. There was elevated Viso in posterior substantia nigra across Parkinsonisms, and Viso, Vic, and ODI were altered in MSAp and PSP in the striatum, globus pallidus, midbrain, thalamus, cerebellum, and corpus callosum relative to controls. The mean effect size across regions for Viso was 0.163, ODI 0.131, Vic 0.122, FW 0.359, and FAt 0.125, with extracellular compartments having the greatest effect size. A key question addressed was if these techniques discriminate PD and atypical Parkinsonism. Both NODDI (AUC: 0.945) and FW imaging (AUC: 0.969) had high accuracy, with no significant difference between models. This study provides new evidence that NODDI and FW imaging offer similar discriminability between PD and atypical Parkinsonism, and FW had higher effect sizes for detecting Parkinsonism within regions across the basal ganglia and cerebellum.
Keywords: Parkinsonism; diffusion MRI; free-water; isotropic volume; neurite density; orientation dispersion.
© 2019 Wiley Periodicals, Inc.
Conflict of interest statement
Dr. Derek B. Archer reports grant support from the Parkinson's Foundation. Dr. Nikolaus R. McFarland reports grants from the NIH and the Michael J. Fox Foundation, and has received personal honoraria from the NIH and the American Academy of Neurology. Dr. Michael S. Okun serves as consultant for the National Parkinson's Foundation, and has received research grants from the National Institutes of Health, National Parkinson's Foundation, Michael J. Fox Foundation, Parkinson Alliance, Smallwood Foundation, Bachmann‐Strauss Foundation, Tourette Syndrome Association, and UF Foundation. Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. Dr. Tanya Simuni reports grants from NINDS, Michael J. Fox Foundation, Parkinson's Foundation, Biogen, Roche, Neuroderm, Sanofi, and Sun Pharma for research and clinical trials, and served as a consultant for Michael J. Fox Foundation, Parkinson's Foundation, Acadia, Abbvie, Adamas, Anavex, Allergan, Accorda, Denali, Neuroderm, Neurocrine, Revance, Sanofi, Sunovion, TEVA, Takeda, Voyager, and US World Meds. Dr. Simuni has received honorarium from Acadia, Adamas, and TEVA. Dr. Cynthia Comella recieves research support from the NIH, Parkinson's Foundation, Dystonia Medical Research Foundation, Merz Pharmaceutical, Revance Therapeutic, Retrophin and Acorda Therapeutic, and has served as a consultant or an advisory committee member for Acorda Therapeutics, Allergan Inc, Lundbeck Ltd., Medtronic Inc., Merz Pharmaceuticals, Acadia Pharmaceuticals, Jazz Pharmaceuticals, Neurocrine Biosciences Inc., Revance Therapeutic, Sunovion. Dr. Comella serves on the editorial board of Clinical Neuropharmacology and Sleep Medicine, and receives royalties from Cambridge, Wolters Kluwer. Dr. Tao Xie has been funded by the Parkinson's Foundation, NIH, Michael J Fox Foundation for Parkinson's Research, Abbvie, Bristol‐Myers Squibb, Biogen and the University of Chicago for research and clinical trials, and also served as consultant for Parkinson's Foundation, Abbvie, and CVS/Caremark. Dr. Daniel M. Corcos reports grants from NIH. Dr. David E. Vaillancourt reports grants from NIH, NSF, and Tyler's Hope Foundation during the conduct of the study, and personal honoraria from NIH and Parkinson's Foundation unrelated to the submitted work. All other authors report no disclosures.
Figures



References
-
- Agosta, F. , Pievani, M. , Svetel, M. , Jecmenica Lukic, M. , Copetti, M. , Tomic, A. , … Filippi, M. (2012). Diffusion tensor MRI contributes to differentiate Richardson's syndrome from PSP‐parkinsonism. Neurobiology of Aging, 33, 2817–2826. - PubMed
-
- Ahmed, Z. , Josephs, K. A. , Gonzalez, J. , DelleDonne, A. , & Dickson, D. W. (2008). Clinical and neuropathologic features of progressive supranuclear palsy with severe pallido‐nigro‐luysial degeneration and axonal dystrophy. Brain, 131, 460–472. - PubMed
-
- Andica, C. , Kamagata, K. , Hatano, T. , Okuzumi, A. , Saito, A. , Nakazawa, M. , … Aoki, S. (2018). Neurite orientation dispersion and density imaging of the nigrostriatal pathway in Parkinson's disease: Retrograde degeneration observed by tract‐profile analysis. Parkinsonism & Related Disorders, 51, 55–60. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

