Characterizing the Feasibility and Performance of Real-World Tumor Progression End Points and Their Association With Overall Survival in a Large Advanced Non-Small-Cell Lung Cancer Data Set
- PMID: 31403818
- PMCID: PMC6873982
- DOI: 10.1200/CCI.19.00013
Characterizing the Feasibility and Performance of Real-World Tumor Progression End Points and Their Association With Overall Survival in a Large Advanced Non-Small-Cell Lung Cancer Data Set
Abstract
Purpose: Large, generalizable real-world data can enhance traditional clinical trial results. The current study evaluates reliability, clinical relevance, and large-scale feasibility for a previously documented method with which to characterize cancer progression outcomes in advanced non-small-cell lung cancer from electronic health record (EHR) data.
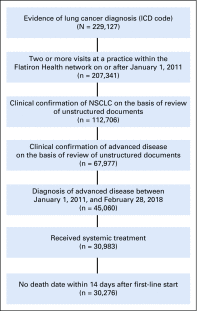
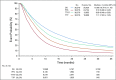
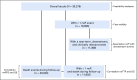
Methods: Patients who were diagnosed with advanced non-small-cell lung cancer between January 1, 2011, and February 28, 2018, with two or more EHR-documented visits and one or more systemic therapy line initiated were identified in Flatiron Health's longitudinal EHR-derived database. After institutional review board approval, we retrospectively characterized real-world progression (rwP) dates, with a random duplicate sample to ascertain interabstractor agreement. We calculated real-world progression-free survival, real-world time to progression, real-world time to next treatment, and overall survival (OS) using the Kaplan-Meier method (index date was the date of first-line therapy initiation), and correlations between OS and other end points were assessed at the patient level (Spearman's ρ).
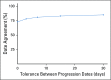
Results: Of 30,276 eligible patients,16,606 (55%) had one or more rwP event. Of these patients, 11,366 (68%) had subsequent death, treatment discontinuation, or new treatment initiation. Correlation of real-world progression-free survival with OS was moderate to high (Spearman's ρ, 0.76; 95% CI, 0.75 to 0.77; evaluable patients, n = 20,020), and for real-world time to progression correlation with OS was lower (Spearman's ρ, 0.69; 95% CI, 0.68 to 0.70; evaluable patients, n = 11,902). Interabstractor agreement on rwP occurrence was 0.94 (duplicate sample, n = 1,065) and on rwP date 0.85 (95% CI, 0.81 to 0.89; evaluable patients n = 358 [patients with two independent event captures within 30 days]). Median rwP abstraction time from individual EHRs was 18.0 minutes (interquartile range, 9.7 to 34.4 minutes).
Conclusion: We demonstrated that rwP-based end points correlate with OS, and that rwP curation from a large, contemporary EHR data set can be reliable, clinically relevant, and feasible on a large scale.
Conflict of interest statement
Sandra D. Griffith
Rebecca A. Miksad
Geoff Calkins
Paul You
Nicole G. Lipitz
Ariel B. Bourla
Erin Williams
Daniel J. George
Deborah Schrag
William B. Capra
Michael D. Taylor
Amy P. Abernethy
No other potential conflicts of interest were reported.
Figures

References
-
- Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: An analysis of 5 years of US Food and Drug Administration approvals. JAMA Intern Med. 2015;175:1992–1994. - PubMed
-
- Wilson MK, Karakasis K, Oza AM. Outcomes and endpoints in trials of cancer treatment: The past, present, and future. Lancet Oncol. 2015;16:e32–e42. - PubMed
-
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

