A DOUBLE-MASKED, RANDOMIZED, SHAM-CONTROLLED, SINGLE-CENTER STUDY WITH PHOTOBIOMODULATION FOR THE TREATMENT OF DRY AGE-RELATED MACULAR DEGENERATION
- PMID: 31404033
- PMCID: PMC7392581
- DOI: 10.1097/IAE.0000000000002632
A DOUBLE-MASKED, RANDOMIZED, SHAM-CONTROLLED, SINGLE-CENTER STUDY WITH PHOTOBIOMODULATION FOR THE TREATMENT OF DRY AGE-RELATED MACULAR DEGENERATION
Abstract
Purpose: The LIGHTSITE I study investigated the efficacy and safety of photobiomodulation (PBM) treatment in subjects with dry age-related macular degeneration.
Methods: Thirty subjects (46 eyes) were treated with the Valeda Light Delivery System, wherein subjects underwent two series of treatments (3× per week for 3-4 weeks) over 1 year. Outcome measures included best-corrected visual acuity, contrast sensitivity, microperimetry, central drusen volume and drusen thickness, and quality of life assessments.
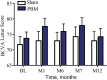
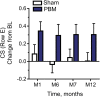
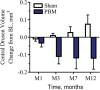
Results: Photobiomodulation-treated subjects showed a best-corrected visual acuity mean letter score gain of 4 letters immediately after each treatment series at Month 1 (M1) and Month 7 (M7). Approximately 50% of PBM-treated subjects showed improvement of ≥5 letters versus 13.6% in sham-treated subjects at M1. High responding subjects (≥5-letter improvement) in the PBM-treated group showed a gain of 8 letters after initial treatment (P < 0.01) and exhibited earlier stages of age-related macular degeneration disease. Statistically significant improvements in contrast sensitivity, central drusen volume, central drusen thickness, and quality of life were observed (P < 0.05). No device-related adverse events were reported.
Conclusion: Photobiomodulation treatment statistically improved clinical and anatomical outcomes with more robust benefits observed in subjects with earlier stages of dry age-related macular degeneration. Repeated PBM treatments are necessary to maintain benefits. These pilot findings support previous reports and suggest the utility of PBM as a safe and effective therapy in subjects with dry age-related macular degeneration.
Conflict of interest statement
R. G. Devenyi and S. N. Markowitz report clinical research agreements and fees from LumiThera Inc, for the conduct of the study. M. R. Munk received lecturer fees from Novartis (Novartis AG) and travel support from Bayer (Bayer AG) and is a consultant for Allergan and Zeiss and reports fees from LumiThera Inc, during the conduct of the study. M. G. Walker and R. Rückert report personal fees and other from LumiThera Inc, during the conduct of the study. LumiThera employees received grant support from National Institutes of Health, National Eye Institute #3R43EY025508 to 01S1. C. E. Tedford and S. E. Stephanie Tedford as well as C. L. Croissant are employees of LumiThera, Inc. The remaining authors have no financial/conflicting interests to disclose.
Figures



References
-
- Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–e116. - PubMed
-
- The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study report number 6. Am J Ophthalmol 2001;132:668–681. - PubMed

