Hantavirus host assemblages and human disease in the Atlantic Forest
- PMID: 31404077
- PMCID: PMC6748440
- DOI: 10.1371/journal.pntd.0007655
Hantavirus host assemblages and human disease in the Atlantic Forest
Abstract
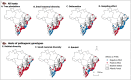
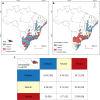
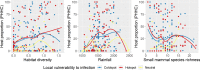
Several viruses from the genus Orthohantavirus are known to cause lethal disease in humans. Sigmodontinae rodents are the main hosts responsible for hantavirus transmission in the tropical forests, savannas, and wetlands of South America. These rodents can shed different hantaviruses, such as the lethal and emerging Araraquara orthohantavirus. Factors that drive variation in host populations may influence hantavirus transmission dynamics within and between populations. Landscape structure, and particularly areas with a predominance of agricultural land and forest remnants, is expected to influence the proportion of hantavirus rodent hosts in the Atlantic Forest rodent community. Here, we tested this using 283 Atlantic Forest rodent capture records and geographically weighted models that allow us to test if predictors vary spatially. We also assessed the correspondence between proportions of hantavirus hosts in rodent communities and a human vulnerability to hantavirus infection index across the entire Atlantic Forest biome. We found that hantavirus host proportions were more positively influenced by landscape diversity than by a particular habitat or agricultural matrix type. Local small mammal diversity also positively influenced known pathogenic hantavirus host proportions, indicating that a plasticity to habitat quality may be more important for these hosts than competition with native forest dwelling species. We found a consistent positive effect of sugarcane and tree plantation on the proportion of rodent hosts, whereas defaunation intensity did not correlate with the proportion of hosts of potentially pathogenic hantavirus genotypes in the community, indicating that non-defaunated areas can also be hotspots for hantavirus disease outbreaks. The spatial match between host hotspots and human disease vulnerability was 17%, while coldspots matched 20%. Overall, we discovered strong spatial and land use change influences on hantavirus hosts at the landscape level across the Atlantic Forest. Our findings suggest disease surveillance must be reinforced in the southern and southeastern regions of the biome where the highest predicted hantavirus host proportion and levels of vulnerability spatially match. Importantly, our analyses suggest there may be more complex rodent community dynamics and interactions with human disease than currently hypothesized.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Impact of Predator Exclusion and Habitat on Seroprevalence of New World Orthohantavirus Harbored by Two Sympatric Rodents within the Interior Atlantic Forest.Viruses. 2021 Sep 29;13(10):1963. doi: 10.3390/v13101963. Viruses. 2021. PMID: 34696393 Free PMC article.
-
Dynamics and drivers of hantavirus prevalence in rodent populations.Vector Borne Zoonotic Dis. 2014 Aug;14(8):537-51. doi: 10.1089/vbz.2013.1562. Vector Borne Zoonotic Dis. 2014. PMID: 25072983 Review.
-
Spatiotemporal Dynamics of Hantavirus Cardiopulmonary Syndrome Transmission Risk in Brazil.Viruses. 2019 Oct 31;11(11):1008. doi: 10.3390/v11111008. Viruses. 2019. PMID: 31683644 Free PMC article.
-
Habitat, species richness and hantaviruses of sigmodontine rodents within the Interior Atlantic Forest, Paraguay.PLoS One. 2018 Aug 1;13(8):e0201307. doi: 10.1371/journal.pone.0201307. eCollection 2018. PLoS One. 2018. PMID: 30067840 Free PMC article.
-
New ecological aspects of hantavirus infection: a change of a paradigm and a challenge of prevention--a review.Virus Genes. 2005 Mar;30(2):157-80. doi: 10.1007/s11262-004-5625-2. Virus Genes. 2005. PMID: 15744574 Review.
Cited by
-
Role of Seaports and Imported Rats in Seoul Hantavirus Circulation, Africa.Emerg Infect Dis. 2023 Jan;29(1):20-25. doi: 10.3201/eid2901.221092. Emerg Infect Dis. 2023. PMID: 36573519 Free PMC article. Review.
-
Impact of Predator Exclusion and Habitat on Seroprevalence of New World Orthohantavirus Harbored by Two Sympatric Rodents within the Interior Atlantic Forest.Viruses. 2021 Sep 29;13(10):1963. doi: 10.3390/v13101963. Viruses. 2021. PMID: 34696393 Free PMC article.
-
Deciphering the Hantavirus Host Range Combining Virology and Species Distribution Models with an Emphasis on the Yellow Pygmy Rice Rat (Oligoryzomys flavescens).Transbound Emerg Dis. 2023 Apr 19;2023:2730050. doi: 10.1155/2023/2730050. eCollection 2023. Transbound Emerg Dis. 2023. PMID: 40303704 Free PMC article.
-
Mixed Effects of Habitat Degradation and Resources on Hantaviruses in Sympatric Wild Rodent Reservoirs within a Neotropical Forest.Viruses. 2021 Jan 9;13(1):85. doi: 10.3390/v13010085. Viruses. 2021. PMID: 33435494 Free PMC article.
-
Genetic and hosts characterization of hantaviruses in port areas in Hainan Province, P. R. China.PLoS One. 2022 Mar 3;17(3):e0264859. doi: 10.1371/journal.pone.0264859. eCollection 2022. PLoS One. 2022. PMID: 35239751 Free PMC article.
References
-
- Gizzi M, Delaere B, Weynand B, Clement J, Maes P, Vergote V, et al. Another case of “european hantavirus pulmonary syndrome” with severe lung, prior to kidney, involvement, and diagnosed by viral inclusions in lung macrophages. Eur J Clin Microbiol Infect Dis. 2013;32: 1341–1345. 10.1007/s10096-013-1885-x - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

