Inducible LGALS3BP/90K activates antiviral innate immune responses by targeting TRAF6 and TRAF3 complex
- PMID: 31404116
- PMCID: PMC6705879
- DOI: 10.1371/journal.ppat.1008002
Inducible LGALS3BP/90K activates antiviral innate immune responses by targeting TRAF6 and TRAF3 complex
Abstract
The galectin 3 binding protein (LGALS3BP, also known as 90K) is a ubiquitous multifunctional secreted glycoprotein originally identified in cancer progression. It remains unclear how 90K functions in innate immunity during viral infections. In this study, we found that viral infections resulted in elevated levels of 90K. Further studies demonstrated that 90K expression suppressed virus replication by inducing IFN and pro-inflammatory cytokine production. Upon investigating the mechanisms behind this event, we found that 90K functions as a scaffold/adaptor protein to interact with TRAF6, TRAF3, TAK1 and TBK1. Furthermore, 90K enhanced TRAF6 and TRAF3 ubiquitination and served as a specific ubiquitination substrate of TRAF6, leading to transcription factor NF-κB, IRF3 and IRF7 translocation from the cytoplasm to the nucleus. Conclusions: 90K is a virus-induced protein capable of binding with the TRAF6 and TRAF3 complex, leading to IFN and pro-inflammatory production.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

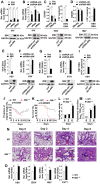




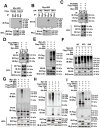
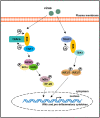
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

