Identification of Mycobacterium tuberculosis Infection in Infants and Children With Partial Discrimination Between Active Disease and Asymptomatic Infection
- PMID: 31404140
- PMCID: PMC6669376
- DOI: 10.3389/fped.2019.00311
Identification of Mycobacterium tuberculosis Infection in Infants and Children With Partial Discrimination Between Active Disease and Asymptomatic Infection
Abstract
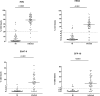
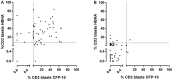
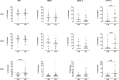
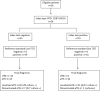
Background: Improved diagnostic tests are needed for the early identification of Mycobacterium tuberculosis-infected young children exposed to an active TB (aTB) index case. We aimed to compare the diagnostic accuracy of new blood-based tests to that of the tuberculin skin test (TST) for the identification of all infected children and for a potential differentiation between aTB and latent TB infection (LTBI). Methods: 144 children exposed to a patient with aTB were included, and those who met all inclusion criteria (130/144) were classified in three groups based on results from classical investigations: non-infected (NI: n = 69, 53%, median age 10 months), LTBI (n = 28, 22%, median age 96 months), aTB disease (n = 33, 25%, median age 24 months). The first whole blood assay consisted of a 7-days in vitro stimulation of blood with four different mycobacterial antigens (40 μl/condition), followed by flow cytometric measurement of the proportions of blast cells appearing among lymphocytes as a result of their specific activation. Thresholds of positivity were determined by Receiver Operating Characteristic (ROC) curve analysis (results of NI children vs. children with LTBI/aTB) in order to identify infected children in a first stage. Other cut-offs were determined to discriminate subgroups of infected children in a second step (results from children with aTB/LTBI). Analysis of blood monocytes and dendritic cell subsets was performed on 100 μl of blood for 25 of these children as a second test in a pilot study. Results: Combining the results of the blast-induced CD3+ T lymphocytes by Heparin-Binding Haemagglutinin and by Culture Filtrate Protein-10 identified all but one infected children (sensitivity 98.2% and specificity 86.9%, compared to 93.4 and 100% for the TST). Further identification among infected children of those with aTB was best achieved by the results of blast-induced CD8+ T lymphocytes by purified protein derivative (sensitivity for localized aTB: 61.9%, specificity 96.3%), whereas high proportions of blood type 2 myeloid dendritic cells (mDC) were a hallmark of LTBI. Conclusions: New blood-based tests requiring a very small volume allow the accurate identification of M. tuberculosis-infected young children among exposed children and are promising to guide the clinical classification of children with aTB or LTBI.
Keywords: FASCIA; Mycobacterium tuberculosis; active tuberculosis; children; dendritic cells; diagnosis; latent infection; lymphoblasts.
Figures

References
-
- WHO Global Tuberculosis Report. WHO (2018). Available online at: http://www.who.int/tb/publications/global_report/en/.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

