ADAR1: "Editor-in-Chief" of Cytoplasmic Innate Immunity
- PMID: 31404141
- PMCID: PMC6669771
- DOI: 10.3389/fimmu.2019.01763
ADAR1: "Editor-in-Chief" of Cytoplasmic Innate Immunity
Abstract
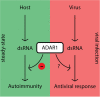
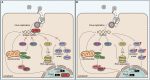
Specialized receptors that recognize molecular patterns such as double stranded RNA duplexes-indicative of viral replication-are potent triggers of the innate immune system. Although their activation is beneficial during viral infection, RNA transcribed from endogenous mobile genetic elements may also act as ligands potentially causing autoimmunity. Recent advances indicate that the adenosine deaminase ADAR1 through RNA editing is involved in dampening the canonical antiviral RIG-I-like receptor-, PKR-, and OAS-RNAse L pathways to prevent autoimmunity. However, this inhibitory effect must be overcome during viral infections. In this review we discuss ADAR1's critical role in balancing immune activation and self-tolerance.
Keywords: ADAR1; MDA5; OAS; PKR; RIG-I; cytoplasmic innate immunity.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

