Clinical trials in Asia: A World Health Organization database study
- PMID: 31404203
- PMCID: PMC6647899
- DOI: 10.4103/picr.PICR_109_18
Clinical trials in Asia: A World Health Organization database study
Abstract
Background and objective: The Asian continent appears to be a growing destination for conducting cost-effective clinical trials, utilizing the available pool of treatment naïve subjects. This study aims to determine the growth rate of clinical trials in Asia.
Methods: A database review was conducted. Registered clinical trials conducted in selected countries in Asia, Europe, Australia, and North America between 2008 and 2017 were searched from the International Clinical Trial Registry Platform. Compound Annual Growth Rate was determined for registered clinical trials.
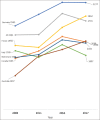
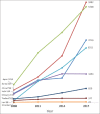
Results: During the 10-year period, a total of 125,918 registered clinical trials were conducted in Asia. There was a 7-fold increase in the number of registered clinical trials in Asia. More trials were registered in Japan than any other Asian country (30.8%). The average annual increase in the number of registered trials was generally higher in Asia than the United States of America, Canada, EU countries, or Australia. Iran recorded the highest average annual increase in the number of all clinical trials in Asia (41.9%). The number of pediatric clinical trials in Iran also increased annually by an average of 30.4%, more than any other country included in this study.
Conclusions: Clinical trials recruitment in Asia is increasing faster than Europe, North America, and Australia. Lower trial cost and large patient pool may be contributory to this increase.
Keywords: Asia; clinical trials; database; pediatric.
Conflict of interest statement
There are no conflicts of interest.
Figures


References
-
- Sullivan F. Asia: Preferred Destination for Clinical Trials-A Frost and Sullivan White Paper. [Last accessed on 2018 Jun 24]. Available from: https://www.novotechcro.com/sites/default/files/170217_FrostSullivan_Asi... .
-
- Thiers FA, Sinskey AJ, Berndt ER. Trends in the globalization of clinical trials. Nat Rev Drug Dis. 2008;7:13–4.

