Safety and Adverse Events after Targeted Lung Denervation for Symptomatic Moderate to Severe Chronic Obstructive Pulmonary Disease (AIRFLOW). A Multicenter Randomized Controlled Clinical Trial
- PMID: 31404499
- PMCID: PMC6909835
- DOI: 10.1164/rccm.201903-0624OC
Safety and Adverse Events after Targeted Lung Denervation for Symptomatic Moderate to Severe Chronic Obstructive Pulmonary Disease (AIRFLOW). A Multicenter Randomized Controlled Clinical Trial
Abstract
Rationale: Targeted lung denervation (TLD) is a bronchoscopic radiofrequency ablation therapy for chronic obstructive pulmonary disease (COPD), which durably disrupts parasympathetic pulmonary nerves to decrease airway resistance and mucus hypersecretion.Objectives: To determine the safety and impact of TLD on respiratory adverse events.Methods: We conducted a multicenter, randomized, sham bronchoscopy-controlled, double-blind trial in patients with symptomatic (modified Medical Research Council dyspnea scale score, ≥2; or COPD Assessment Test score, ≥10) COPD (FEV1, 30-60% predicted). The primary endpoint was the rate of respiratory adverse events between 3 and 6.5 months after randomization (defined as COPD exacerbation, tachypnea, wheezing, worsening bronchitis, worsening dyspnea, influenza, pneumonia, other respiratory infections, respiratory failure, or airway effects requiring therapeutic intervention). Blinding was maintained through 12.5 months.Measurements and Main Results: Eighty-two patients (50% female; mean ± SD: age, 63.7 ± 6.8 yr; FEV1, 41.6 ± 7.3% predicted; modified Medical Research Council dyspnea scale score, 2.2 ± 0.7; COPD Assessment Test score, 18.4 ± 6.1) were randomized 1:1. During the predefined 3- to 6.5-month window, patients in the TLD group experienced significantly fewer respiratory adverse events than those in the sham group (32% vs. 71%, P = 0.008; odds ratio, 0.19; 95% confidence interval, 0.0750-0.4923, P = 0.0006). Between 0 and 12.5 months, these findings were not different (83% vs. 90%; P = 0.52). The risk of COPD exacerbation requiring hospitalization in the 0- to 12.5-month window was significantly lower in the TLD group than in the sham group (hazard ratio, 0.35; 95% confidence interval, 0.13-0.99; P = 0.039). There was no statistical difference in the time to first moderate or severe COPD exacerbation, patient-reported symptoms, or other physiologic measures over the 12.5 months of follow-up.Conclusions: Patients with symptomatic COPD treated with TLD combined with optimal pharmacotherapy had fewer study-defined respiratory adverse events, including hospitalizations for COPD exacerbation.Clinical trial registered with www.clinicaltrials.gov (NCT02058459).
Keywords: anticholinergic; bronchoscopy; chronic obstructive pulmonary disease; nerves; targeted lung denervation.
Figures
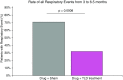
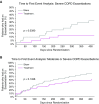
Comment in
-
Calming Nervous Airways: Targeted Lung Denervation for Chronic Obstructive Pulmonary Disease.Am J Respir Crit Care Med. 2019 Dec 15;200(12):1455-1456. doi: 10.1164/rccm.201908-1586ED. Am J Respir Crit Care Med. 2019. PMID: 31469579 Free PMC article. No abstract available.
References
-
- Albert P, Agusti A, Edwards L, Tal-Singer R, Yates J, Bakke P, et al. Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax. 2012;67:701–708. - PubMed
-
- Klooster K, ten Hacken NH, Hartman JE, Kerstjens HA, van Rikxoort EM, Slebos DJ. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med. 2015;373:2325–2335. - PubMed
-
- Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, et al. IMPACT Investigators. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–1680. - PubMed
-
- Pavord ID, Chanez P, Criner GJ, Kerstjens HAM, Korn S, Lugogo N, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377:1613–1629. - PubMed
-
- Chapman KR, Hurst JR, Frent SM, Larbig M, Fogel R, Guerin T, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198:329–339. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

