Impact of oral metronidazole treatment on the vaginal microbiota and correlates of treatment failure
- PMID: 31404542
- PMCID: PMC6995998
- DOI: 10.1016/j.ajog.2019.08.008
Impact of oral metronidazole treatment on the vaginal microbiota and correlates of treatment failure
Abstract
Background: Metronidazole is the first-line treatment for bacterial vaginosis, but cure rates are suboptimal and recurrence rates high.
Objectives: To evaluate the impact of a standard course of oral metronidazole treatment (500 mg twice per day for 7 days) on the vaginal microbiota of Rwandan bacterial vaginosis patients using microscopy and 16S rRNA gene sequencing, and to evaluate correlates of treatment failure.
Study design: HIV-negative, nonpregnant women aged 18-45 years with bacterial vaginosis and/or Trichomonas vaginalis (N=68) were interviewed and sampled before and after metronidazole treatment. They were also screened, and treated if applicable, for other urogenital infections. The vaginal microbiota was assessed by Gram stain Nugent scoring, Illumina 16S rRNA HiSeq sequencing (relative abundances), and BactQuant 16S gene quantitative polymerase chain reaction (estimated concentrations). Only women with a pretreatment Nugent score of 7-10 and a valid posttreatment Nugent score (N=55) were included in metronidazole treatment failure analyses, with treatment failure defined as a posttreatment Nugent score of 4-10.
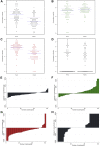
Results: The bacterial vaginosis cure rate by Nugent scoring was 54.5%. The mean total vaginal bacterial concentration declined from 6.59 to 5.85 log10/μL (P<.001), which was mostly due to a reduction in mean bacterial vaginosis-associated anaerobes concentration (all bacterial vaginosis-associated anaerobe taxa combined) from 6.23 to 4.55 log10/μL (P<.001). However, only 16.4% of women had a bacterial vaginosis anaerobes concentration reduction of more than 50%, and only 3 women had complete eradication. The mean concentration of lactobacilli (all species combined) increased from 4.98 to 5.56 log10/μL (P=.017), with L. iners being the most common species pre- and posttreatment. The mean concentration of pathobionts (defined as Proteobacteria, streptococci, staphylococci, enterococci, and a few others) did not change significantly: from 1.92 log10/μL pretreatment to 2.01 log10/μL posttreatment (P=.939). Pretreatment pathobionts concentration, and having a pretreatment vaginal microbiota type containing more than 50% Gardnerella vaginalis (compared with less than 50%), were associated with increased likelihood of treatment failure, but the latter did not reach statistical significance (P=.044 and P=.084, respectively).
Conclusions: Metronidazole alone may not cure women with high G. vaginalis relative abundance, potentially due to biofilm presence, and women with high pathobionts concentration. These women may benefit from additional biofilm-disrupting and/or pathobiont-targeting treatments.
Trial registration: ClinicalTrials.gov NCT02459665.
Keywords: 16S rRNA gene sequencing; Lactobacilli; anaerobes; antibiotics; bacterial vaginosis; biofilm; metronidazole; trichomoniasis; vaginal dysbiosis; vaginal microbiota.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- van de Wijgert J.H., Jespers V. Incorporating microbiota data into epidemiologic models: examples from vaginal microbiota research. Ann Epidemiol. 2016;26:360–365. - PubMed
-
- van de Wijgert J.H.H.M., Morrison C.S., Cornelisse P.G.A. Bacterial vaginosis and vaginal yeast, but not vaginal cleansing, increase HIV-1 acquisition in African women. J Acquir Immune Defic Syndr. 2008;48:203–210. - PubMed
-
- Taylor B.D., Darville T., Haggerty C.L. Does bacterial vaginosis cause pelvic inflammatory disease? Sex Transm Dis. 2013;40:117–122. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

