Microfluidic Generation of Amino-Functionalized Hydrogel Microbeads Capable of On-Bead Bioassay
- PMID: 31405057
- PMCID: PMC6723060
- DOI: 10.3390/mi10080527
Microfluidic Generation of Amino-Functionalized Hydrogel Microbeads Capable of On-Bead Bioassay
Abstract
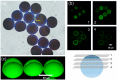
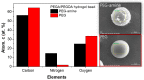
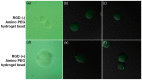
Microfluidic generation of hydrogel microbeads is a highly efficient and reproducible approach to create various functional hydrogel beads. Here, we report a method to prepare crosslinked amino-functionalized polyethylene glycol (PEG) microbeads using a microfluidic channel. The microbeads generated from a microfluidic device were evaluated by scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS) and confocal laser scanning microscopy, respectively. We found that the microbeads were monodisperse and the amino groups were localized on the shell region of the microbeads. A swelling test exhibited compatibility with various solvents. A cell binding assay was successfully performed with RGD peptide-coupled amino-functionalized hydrogel microbeads. This strategy will enable the large production of the various functional microbeads, which can be used for solid phase peptide synthesis and on-bead bioassays.
Keywords: hydrogel microbead; microfluidic channel; on-bead bioassay; peptide synthesis; polyethylene glycol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Utilizing microfluidics to synthesize polyethylene glycol microbeads for Förster resonance energy transfer based glucose sensing.Biomicrofluidics. 2012 Jun;6(2):22006-220069. doi: 10.1063/1.3694869. Epub 2012 Apr 6. Biomicrofluidics. 2012. PMID: 22655010 Free PMC article.
-
Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification.J Vis Exp. 2019 Apr 30;(146). doi: 10.3791/6328. J Vis Exp. 2019. PMID: 31038480
-
Adsorption and desorption of DNA-functionalized beads in glass microfluidic channels.Biomicrofluidics. 2019 Sep 30;13(5):054104. doi: 10.1063/1.5115160. eCollection 2019 Sep. Biomicrofluidics. 2019. PMID: 31592058 Free PMC article.
-
The application of microbeads to microfluidic systems for enhanced detection and purification of biomolecules.Methods. 2017 Mar 1;116:112-124. doi: 10.1016/j.ymeth.2016.12.005. Epub 2016 Dec 10. Methods. 2017. PMID: 27965121 Review.
-
Bead-based microfluidic immunoassays: the next generation.Biosens Bioelectron. 2007 Feb 15;22(7):1197-204. doi: 10.1016/j.bios.2006.06.005. Epub 2006 Jul 20. Biosens Bioelectron. 2007. PMID: 16857357 Review.
Cited by
-
Editorial for the Special Issue on Microfluidics for Soft Matter and Mechanobiology.Micromachines (Basel). 2020 Apr 2;11(4):372. doi: 10.3390/mi11040372. Micromachines (Basel). 2020. PMID: 32252304 Free PMC article.
-
Probing Single-Cell Macrophage Polarization and Heterogeneity Using Thermo-Reversible Hydrogels in Droplet-Based Microfluidics.Front Bioeng Biotechnol. 2021 Oct 14;9:715408. doi: 10.3389/fbioe.2021.715408. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34722475 Free PMC article.
-
Hydrogels for Single-Cell Microgel Production: Recent Advances and Applications.Front Bioeng Biotechnol. 2022 Jun 17;10:891461. doi: 10.3389/fbioe.2022.891461. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35782502 Free PMC article. Review.
References
-
- Gitsov I., Zhu C. Amphiphilic Hydrogels Constructed by Poly(ethylene glycol) and Shape-Persistent Dendritic Fragments. Macromolecules. 2002;35:8418–8427. doi: 10.1021/ma020935v. - DOI
-
- Palomo J.M. Solid-phase peptide synthesis: An overview focused on the preparation of biologically relevant peptides. RSC Adv. 2014;4:32658–32672. doi: 10.1039/C4RA02458C. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

