N-Acetylcysteine Rinse for Thick Secretion and Mucositis of Head and Neck Chemoradiotherapy (Alliance MC13C2): A Double-Blind Randomized Clinical Trial
- PMID: 31405750
- PMCID: PMC6742495
- DOI: 10.1016/j.mayocp.2019.03.031
N-Acetylcysteine Rinse for Thick Secretion and Mucositis of Head and Neck Chemoradiotherapy (Alliance MC13C2): A Double-Blind Randomized Clinical Trial
Abstract
Objective: To determine whether N-acetylcysteine rinse was safe and could improve thickened secretions and dry mouth during and after radiotherapy.
Patients and methods: We designed a prospective pilot double-blind, placebo-controlled randomized clinical trial (Alliance MC13C2). Adult patients (age ≥18 years) were enrolled if they underwent chemoradiotherapy (≥60 Gy). Patients initiated testing rinse within 3 days of starting radiotherapy. With swish-and-spit, they received 10% N-acetylcysteine (2500 mg daily) or placebo rinse solution 5 times daily during radiotherapy and 2 weeks postradiotherapy. The primary aim was to evaluate N-acetylcysteine in improvement of saliva viscosity with the Groningen Radiotherapy-Induced Xerostomia questionnaire. Secondary aims included evaluating xerostomia improvement by the same questionnaire and with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Head and Neck-35 Questions survey and adverse-event profiles. The type I error rate was 20%.
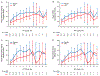
Results: Thirty-two patients undergoing chemoradiotherapy were enrolled. Baseline characteristics were balanced for placebo (n=17) and N-acetylcysteine (n=15). N-acetylcysteine was better for improving sticky saliva (area under curve, P=.12). Scores of multiple secondary end points favored N-acetylcysteine, including sticky saliva daytime (P=.04), daytime and total xerostomia (both P=.02), pain (P=.18), and trouble with social eating (P=.15). Repeated measures models confirmed the findings. Taste was a major dissatisifer for N-acetylcysteine rinse; however, both testing rinses were safe and well tolerated overall.
Conclusion: Our pilot data showed that N-acetylcysteine rinse was safe and provided strong evidence of potential efficacy for improving thickened saliva and xerostomia by patient-reported outcome. A confirmatory phase 3 trial is required.
Trial registration: clinicaltrials.gov Identifier: NCT02123511.
Copyright © 2019 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J. Clin. Oncol 2003;21:92–98. - PubMed
-
- Denis F, Garaud P, Bardet E, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J. Clin. Oncol 2004;22:69–76. - PubMed
-
- O’Neill M, Heron DE, Flickinger JC, Smith R, Ferris RL, Gibson M. Posttreatment quality-of-life assessment in patients with head and neck cancer treated with intensity-modulated radiation therapy. Am. J. Clin. Oncol 2011;34:478–482. - PubMed
-
- Jaguar GC, Prado JD, Campanhã D, Alves FA. Clinical features and preventive therapies of radiation-induced xerostomia in head and neck cancer patient: a literature review. Applied Cancer Research. 2017;37:31.
-
- Aihara M, Dobashi K, Akiyama M, Naruse I, Nakazawa T, Mori M. Effects of N-acetylcysteine and ambroxol on the production of IL-12 and IL-10 in human alveolar macrophages. Respiration. 2000;67:662–671. - PubMed

