Population Pharmacokinetics of the Antituberculosis Agent Pretomanid
- PMID: 31405856
- PMCID: PMC6761531
- DOI: 10.1128/AAC.00907-19
Population Pharmacokinetics of the Antituberculosis Agent Pretomanid
Abstract
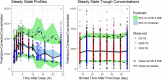
A population pharmacokinetic (PopPK) model for pretomanid was developed using data from 14 studies in the pretomanid development program: six phase 1 studies, six phase 2 studies, and two phase 3 studies. The final analysis data set contained 17,725 observations from 1,054 subjects, including healthy subjects and subjects with drug-sensitive, multidrug-resistant, or extensively drug-resistant pulmonary tuberculosis dosed pretomanid in monotherapy or combination therapy for up to 6 months. Pretomanid pharmacokinetic behavior was described by a one-compartment model that at a given dose was linear in its absorption and clearance processes but where the rate of absorption and extent of bioavailability changed with dose. Clearance and volume of distribution scaled allometrically with weight. Apparent clearance in females was 18% less than in males. Among HIV-positive subjects, absent the effect of CYP3A4-inducing antiretrovirals, apparent clearance was 6% higher. Some effects of total bilirubin and albumin were found, but the impacts on exposure were small. Bioavailability in the fasted condition was about half that in the fed condition. Relative bioavailability decreased with increasing dose in the fasted condition, but not for doses of ≤200 mg in the fed condition. HIV-positive subjects taking efavirenz and lopinavir/ritonavir had exposures that were reduced by 46 and 17%, respectively. There was little evidence for noteworthy effects of regimen partners on pretomanid. Standard diagnostics indicated that the model described the voluminous, diverse data well, so that the model could be used to generate exposure metrics for exposure/response analyses to be reported elsewhere.
Keywords: Mycobacterium tuberculosis; antibiotic resistance; multidrug resistance; tuberculosis.
Copyright © 2019 Salinger et al.
Figures

References
-
- World Health Organization. 2018. Global tuberculosis report—2018. World Health Organization, Geneva, Switzerland: https://www.who.int/tb/publications/global_report/en/.
-
- Conradie F, Diacon A, Everitt D, Mendel C, Crook A, Howell P, Comins K, Spigelman M. 2018. Sustained high rate of successful treatment outcomes: interim results of 75 patients in the Nix-TB clinical study of pretomanid, bedaquiline and linezolid. 49th Abstracts of the World Conference on Lung Health of the International Union Against Tuberculosis and Lung Disease, abstr OA03-213-215 https://www.abstractserver.com/TheUnion2018/TheUnion2018_Abstracts_Web.pdf.
-
- Dawson R, Harris K, Conradie A, Burger D, Murray S, Mendel C, Spigelman M. 2017. Efficacy of bedaquiline, pretomanid, moxifloxacin, and PZA (BPaMZ) against DS- and MDR-TB. Abstracts of the Conference on Rotoviruses and Opportunistic Infections, abstr 724-LB http://www.croiconference.org/sessions/efficacy-bedaquiline-pretomanid-m....
-
- International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use. 2019. ICH, Geneva, Switzerland: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Ef.... Accessed 12 July 2019.
-
- FDA. 2019. Guidance for industry: population pharmacokinetics. U.S. Department of Health and Human Services/Food and Drug Administration/Center for Drug Evaluation and Research, Bethesda, MD.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

