Regulation of photoprotection gene expression in Chlamydomonas by a putative E3 ubiquitin ligase complex and a homolog of CONSTANS
- PMID: 31405963
- PMCID: PMC6717296
- DOI: 10.1073/pnas.1821689116
Regulation of photoprotection gene expression in Chlamydomonas by a putative E3 ubiquitin ligase complex and a homolog of CONSTANS
Abstract
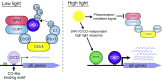
Photosynthetic organisms use nonphotochemical quenching (NPQ) mechanisms to dissipate excess absorbed light energy and protect themselves from photooxidation. In the model green alga Chlamydomonas reinhardtii, the capacity for rapidly reversible NPQ (qE) is induced by high light, blue light, and UV light via increased expression of LHCSR and PSBS genes that are necessary for qE. Here, we used a forward genetics approach to identify SPA1 and CUL4, components of a putative green algal E3 ubiquitin ligase complex, as critical factors in a signaling pathway that controls light-regulated expression of the LHCSR and PSBS genes in C. reinhardtii The spa1 and cul4 mutants accumulate increased levels of LHCSR1 and PSBS proteins in high light, and unlike the wild type, they express LHCSR1 and exhibit qE capacity even when grown in low light. The spa1-1 mutation resulted in constitutively high expression of LHCSR and PSBS RNAs in both low light and high light. The qE and gene expression phenotypes of spa1-1 are blocked by mutation of CrCO, a B-box Zn-finger transcription factor that is a homolog of CONSTANS, which controls flowering time in plants. CONSTANS-like cis-regulatory sequences were identified proximal to the qE genes, consistent with CrCO acting as a direct activator of qE gene expression. We conclude that SPA1 and CUL4 are components of a conserved E3 ubiquitin ligase that acts upstream of CrCO, whose regulatory function is wired differently in C. reinhardtii to control qE capacity via cis-regulatory CrCO-binding sites at key photoprotection genes.
Keywords: light harvesting; light signaling; nonphotochemical quenching; photomorphogenesis; photosynthesis.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
The CONSTANS flowering complex controls the protective response of photosynthesis in the green alga Chlamydomonas.Nat Commun. 2019 Sep 10;10(1):4099. doi: 10.1038/s41467-019-11989-x. Nat Commun. 2019. PMID: 31506429 Free PMC article.
-
UV-B photoreceptor-mediated protection of the photosynthetic machinery in Chlamydomonas reinhardtii.Proc Natl Acad Sci U S A. 2016 Dec 20;113(51):14864-14869. doi: 10.1073/pnas.1607695114. Epub 2016 Dec 5. Proc Natl Acad Sci U S A. 2016. PMID: 27930292 Free PMC article.
-
Abscisic acid enhances non-photochemical quenching through SnRK2 and ABI3 in Physcomitrium patens.J Plant Res. 2025 Jul;138(4):625-636. doi: 10.1007/s10265-025-01627-7. Epub 2025 Apr 7. J Plant Res. 2025. PMID: 40192897
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
Cited by
-
Regulation of Microalgal Photosynthetic Electron Transfer.Plants (Basel). 2024 Jul 29;13(15):2103. doi: 10.3390/plants13152103. Plants (Basel). 2024. PMID: 39124221 Free PMC article. Review.
-
Macroscale structural changes of thylakoid architecture during high light acclimation in Chlamydomonas reinhardtii.Photosynth Res. 2024 Dec;162(2-3):427-437. doi: 10.1007/s11120-023-01067-1. Epub 2024 Jan 5. Photosynth Res. 2024. PMID: 38180578 Free PMC article.
-
Time series single-cell transcriptional atlases reveal cell fate differentiation driven by light in Arabidopsis seedlings.Nat Plants. 2023 Dec;9(12):2095-2109. doi: 10.1038/s41477-023-01544-4. Epub 2023 Oct 30. Nat Plants. 2023. PMID: 37903986 Free PMC article.
-
Potential abiotic stress targets for modern genetic manipulation.Plant Cell. 2023 Jan 2;35(1):139-161. doi: 10.1093/plcell/koac327. Plant Cell. 2023. PMID: 36377770 Free PMC article. Review.
-
COP1 controls salt stress tolerance by modulating sucrose content.Plant Signal Behav. 2022 Dec 31;17(1):2096784. doi: 10.1080/15592324.2022.2096784. Plant Signal Behav. 2022. PMID: 35833514 Free PMC article.
References
-
- Baker N. R., Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 59, 89–113 (2008). - PubMed
-
- Horton P., Ruban A. V., Walters R. G., Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 655–684 (1996). - PubMed
-
- Briantais J.-M., Vernotte C., Picaud M., Krause G. H., A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts. Biochim. Biophys. Acta 548, 128–138 (1979). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

