Prebiotic amino acids bind to and stabilize prebiotic fatty acid membranes
- PMID: 31405964
- PMCID: PMC6717294
- DOI: 10.1073/pnas.1900275116
Prebiotic amino acids bind to and stabilize prebiotic fatty acid membranes
Abstract
The membranes of the first protocells on the early Earth were likely self-assembled from fatty acids. A major challenge in understanding how protocells could have arisen and withstood changes in their environment is that fatty acid membranes are unstable in solutions containing high concentrations of salt (such as would have been prevalent in early oceans) or divalent cations (which would have been required for RNA catalysis). To test whether the inclusion of amino acids addresses this problem, we coupled direct techniques of cryoelectron microscopy and fluorescence microscopy with techniques of NMR spectroscopy, centrifuge filtration assays, and turbidity measurements. We find that a set of unmodified, prebiotic amino acids binds to prebiotic fatty acid membranes and that a subset stabilizes membranes in the presence of salt and Mg2+ Furthermore, we find that final concentrations of the amino acids need not be high to cause these effects; membrane stabilization persists after dilution as would have occurred during the rehydration of dried or partially dried pools. In addition to providing a means to stabilize protocell membranes, our results address the challenge of explaining how proteins could have become colocalized with membranes. Amino acids are the building blocks of proteins, and our results are consistent with a positive feedback loop in which amino acids bound to self-assembled fatty acid membranes, resulting in membrane stabilization and leading to more binding in turn. High local concentrations of molecular building blocks at the surface of fatty acid membranes may have aided the eventual formation of proteins.
Keywords: amino acid; membrane; origin of life; prebiotic; protocell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


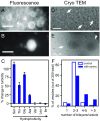
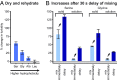
Similar articles
-
Binding of Dipeptides to Fatty Acid Membranes Explains Their Colocalization in Protocells but Does Not Select for Them Relative to Unjoined Amino Acids.J Phys Chem B. 2021 Jul 29;125(29):7933-7939. doi: 10.1021/acs.jpcb.1c01485. Epub 2021 Jul 20. J Phys Chem B. 2021. PMID: 34283913 Free PMC article.
-
A Step toward Molecular Evolution of RNA: Ribose Binds to Prebiotic Fatty Acid Membranes, and Nucleosides Bind Better than Individual Bases Do.Chembiochem. 2020 Oct 1;21(19):2764-2767. doi: 10.1002/cbic.202000260. Epub 2020 Jun 5. Chembiochem. 2020. PMID: 32358921 Free PMC article.
-
Prebiotic Protocell Model Based on Dynamic Protein Membranes Accommodating Anabolic Reactions.Langmuir. 2019 Jul 23;35(29):9593-9610. doi: 10.1021/acs.langmuir.9b00445. Epub 2019 Jul 9. Langmuir. 2019. PMID: 31287709
-
A Self-Assembled Aggregate Composed of a Fatty Acid Membrane and the Building Blocks of Biological Polymers Provides a First Step in the Emergence of Protocells.Life (Basel). 2016 Aug 11;6(3):33. doi: 10.3390/life6030033. Life (Basel). 2016. PMID: 27529283 Free PMC article. Review.
-
The origins of cellular life.Cold Spring Harb Perspect Biol. 2010 Sep;2(9):a002212. doi: 10.1101/cshperspect.a002212. Epub 2010 May 19. Cold Spring Harb Perspect Biol. 2010. PMID: 20484387 Free PMC article. Review.
Cited by
-
The astrochemical evolutionary traits of phospholipid membrane homochirality.Nat Rev Chem. 2024 Sep;8(9):652-664. doi: 10.1038/s41570-024-00627-w. Epub 2024 Jul 18. Nat Rev Chem. 2024. PMID: 39025922 Review.
-
Origin of Life on Mars: Suitability and Opportunities.Life (Basel). 2021 Jun 9;11(6):539. doi: 10.3390/life11060539. Life (Basel). 2021. PMID: 34207658 Free PMC article.
-
The Way forward for the Origin of Life: Prions and Prion-Like Molecules First Hypothesis.Life (Basel). 2021 Aug 25;11(9):872. doi: 10.3390/life11090872. Life (Basel). 2021. PMID: 34575021 Free PMC article.
-
Catalytic Metal Ion-Substrate Coordination during Nonenzymatic RNA Primer Extension.J Am Chem Soc. 2024 Apr 17;146(15):10632-10639. doi: 10.1021/jacs.4c00323. Epub 2024 Apr 5. J Am Chem Soc. 2024. PMID: 38579124 Free PMC article.
-
Lipid and Lipidation in Membrane Fusion.J Membr Biol. 2022 Dec;255(6):691-703. doi: 10.1007/s00232-022-00267-5. Epub 2022 Sep 14. J Membr Biol. 2022. PMID: 36102950 Free PMC article. Review.
References
-
- Deamer D., Dworkin J. P., Sandford S. A., Bernstein M. P., Allamandola L. J., The first cell membranes. Astrobiology 2, 371–381 (2002). - PubMed
-
- Morigaki K., Walde P., Fatty acid vesicles. Curr. Opin. Coll. Int. Sci. 12, 75–80 (2007).
-
- Proskurowski G., et al. , Abiogenic hydrocarbon production at lost city hydrothermal field. Science 319, 604–607 (2008). - PubMed
-
- Lawless J., Yuen G., Quantification of monocarboxylic acids in the Murchison carbonaceous meteorite. Nature 282, 396–398 (1979).
-
- Monnard P.-A., Apel C. L., Kanavarioti A., Deamer D. W., Influence of ionic inorganic solutes on self-assembly and polymerization processes related to early forms of life: Implications for a prebiotic aqueous medium. Astrobiology 2, 139–152 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

