A ubiquitin-like domain controls protein kinase D dimerization and activation by trans-autophosphorylation
- PMID: 31406020
- PMCID: PMC6768651
- DOI: 10.1074/jbc.RA119.008713
A ubiquitin-like domain controls protein kinase D dimerization and activation by trans-autophosphorylation
Abstract
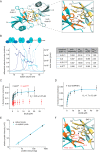
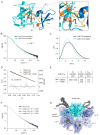
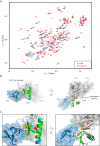
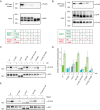
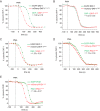
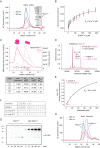
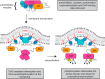
Protein kinase D (PKD) is an essential Ser/Thr kinase in animals and controls a variety of diverse cellular functions, including vesicle trafficking and mitogenesis. PKD is activated by recruitment to membranes containing the lipid second messenger diacylglycerol (DAG) and subsequent phosphorylation of its activation loop. Here, we report the crystal structure of the PKD N terminus at 2.2 Å resolution containing a previously unannotated ubiquitin-like domain (ULD), which serves as a dimerization domain. A single point mutation in the dimerization interface of the ULD not only abrogated dimerization in cells but also prevented PKD activation loop phosphorylation upon DAG production. We further show that the kinase domain of PKD dimerizes in a concentration-dependent manner and autophosphorylates on a single residue in its activation loop. We also provide evidence that PKD is expressed at concentrations 2 orders of magnitude below the ULD dissociation constant in mammalian cells. We therefore propose a new model for PKD activation in which the production of DAG leads to the local accumulation of PKD at the membrane, which drives ULD-mediated dimerization and subsequent trans-autophosphorylation of the kinase domain.
Keywords: autophosphorylation; crystal structure; diacylglycerol; dimerization; protein kinase D (PKD); second messenger; signal transduction; structural biology; ubiquitin-like domain.
© 2019 Elsner et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Sinnett-Smith J., Jacamo R., Kui R., Wang Y. M., Young S. H., Rey O., Waldron R. T., and Rozengurt E. (2009) Protein kinase D mediates mitogenic signaling by Gq-coupled receptors through protein kinase C-independent regulation of activation loop Ser744 and Ser748 phosphorylation. J. Biol. Chem. 284, 13434–13445 10.1074/jbc.M806554200 - DOI - PMC - PubMed
-
- Sumara G., Formentini I., Collins S., Sumara I., Windak R., Bodenmiller B., Ramracheya R., Caille D., Jiang H., Platt K. A., Meda P., Aebersold R., Rorsman P., and Ricci R. (2009) Regulation of PKD by the MAPK p38δ in insulin secretion and glucose homeostasis. Cell 136, 235–248 10.1016/j.cell.2008.11.018 - DOI - PMC - PubMed
-
- Zhang Z., Meszaros G., He W.-T., Xu Y., de Fatima Magliarelli H., Mailly L., Mihlan M., Liu Y., Puig Gámez M., Goginashvili A., Pasquier A., Bielska O., Neven B., Quartier P., Aebersold R., et al. (2017) Protein kinase D at the Golgi controls NLRP3 inflammasome activation. J. Exp. Med. 214, 2671–2693 10.1084/jem.20162040 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

