Band-collision gel electrophoresis
- PMID: 31406115
- PMCID: PMC6690962
- DOI: 10.1038/s41467-019-11438-9
Band-collision gel electrophoresis
Abstract
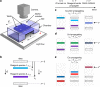
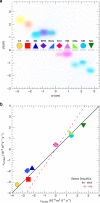
Electrophoretic mobility shift assays are widely used in gel electrophoresis to study binding interactions between different molecular species loaded into the same well. However, shift assays can access only a subset of reaction possibilities that could be otherwise seen if separate bands of reagent species might instead be collisionally reacted. Here, we adapt gel electrophoresis by fabricating two or more wells in the same lane, loading these wells with different reagent species, and applying an electric field, thereby producing collisional reactions between propagating pulse-like bands of these species, which we image optically. For certain pairs of anionic and cationic dyes, propagating bands pass through each other unperturbed; yet, for other pairs, we observe complexing and precipitation reactions, indicating strong attractive interactions. We generalize this band-collision gel electrophoresis (BCGE) approach to other reaction types, including acid-base, ligand exchange, and redox, as well as to colloidal species in passivated large-pore gels.
Conflict of interest statement
There is a pending and at present unlicensed U.S. provisional patent application assigned to and filed by UCLA relating to this work.
Figures





References
-
- Casse F, Boucher C, Julliot JS, Michel M, Denarie J. Identification and characterization of large plasmids in Rhizobium meliloti using agarose gel electrophoresis. J. Gen. Microbiol. 1979;113:229–242. doi: 10.1099/00221287-113-2-229. - DOI

