Atorvastatin increases the production of proinflammatory cytokines and decreases the survival of Escherichia coli-infected mice
- PMID: 31406240
- PMCID: PMC6690901
- DOI: 10.1038/s41598-019-48282-2
Atorvastatin increases the production of proinflammatory cytokines and decreases the survival of Escherichia coli-infected mice
Abstract
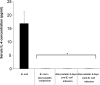
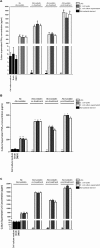
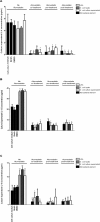
To assess whether the immunosuppressive effects of atorvastatin outweigh its antibacterial ones in an infection, mice were infected with Escherichia coli and administered atorvastatin; survival rates were then monitored. Mice treated with atorvastatin post-infection showed a remarkable decrease in their survival rate. On the other hand, the higher the level of serum IFN-γ in the infected mice treated with atorvastatin, the lower was the survival rate. Levels of IL-4 were markedly depressed in all groups infected with E. coli and treated with atorvastatin. Since atorvastatin inhibits IFN-γ expression in the absence of bacterial infection, we examined whether bacterial lipopolysaccharide (LPS) was the element capable of overriding this inhibition. Mouse peripheral blood mononuclear cells were treated with atorvastatin and lipopolysaccharide ex vivo then proinflammatory (IFN-γ, TNFα, IL-6) and prohumoral/regulatory (IL-4, IL-13, IL-10) cytokine levels were analyzed in culture supernatants. While proinflammatory cytokine levels were decreased upon treatment with atorvastatin alone, their levels were markedly elevated by treatment with LPS, bacterial lysate or bacterial culture supernatant. On the other hand, atorvastatin exerted an inhibitory effect on production of the prohumoral/regulatory cytokines. Our data indicates that any consideration for statins as antimicrobial treatment should assess the possible adverse outcomes.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

