A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients
- PMID: 31406321
- PMCID: PMC7000322
- DOI: 10.1038/s41436-019-0633-8
A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients
Abstract
Purpose: Despite the rapid uptake of multigene panel testing (MGPT) for hereditary cancer predisposition, there is limited guidance surrounding indications for testing and genes to include.
Methods: To inform the clinical approach to hereditary cancer MGPT, we comprehensively evaluated 32 cancer predisposition genes by assessing phenotype-specific pathogenic variant (PV) frequencies, cancer risk associations, and performance of genetic testing criteria in a cohort of 165,000 patients referred for MGPT.
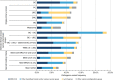
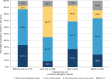
Results: We identified extensive genetic heterogeneity surrounding predisposition to cancer types commonly referred for germline testing (breast, ovarian, colorectal, uterine/endometrial, pancreatic, and melanoma). PV frequencies were highest among patients with ovarian cancer (13.8%) and lowest among patients with melanoma (8.1%). Fewer than half of PVs identified in patients meeting testing criteria for only BRCA1/2 or only Lynch syndrome occurred in the respective genes (33.1% and 46.2%). In addition, 5.8% of patients with PVs in BRCA1/2 and 26.9% of patients with PVs in Lynch syndrome genes did not meet respective testing criteria.
Conclusion: Opportunities to improve upon identification of patients at risk for hereditary cancer predisposition include revising BRCA1/2 and Lynch syndrome testing criteria to include additional clinically actionable genes with overlapping phenotypes and relaxing testing criteria for associated cancers.
Keywords: cancer predisposition; clinical validity; hereditary cancer; multigene panel; testing criteria.
Figures

References
-
- Lowstuter K, Espenschied CR, Sturgeon D, et al. Unexpected CDH1 mutations identified on multigene panels pose clinical management challenges. JCO Precis Oncol. 2017;1:1–12. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

