Tumor mutation burden: from comprehensive mutational screening to the clinic
- PMID: 31406485
- PMCID: PMC6686509
- DOI: 10.1186/s12935-019-0929-4
Tumor mutation burden: from comprehensive mutational screening to the clinic
Abstract
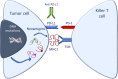
The recent advent of immunomodulatory therapies into the clinic has demanded the identification of innovative predictive biomarkers to identify patients most likely to respond to immunotherapy and support the design of tailored clinical trials. Current molecular testing for selection of patients with gastrointestinal or pulmonary carcinomas relies on the prevalence of PD-L1 expression in tumor as well as immune cells by immunohistochemistry and/or on the evaluation of the microsatellite status. Tumor Mutational Burden (TMB) has emerged as a promising novel biomarker in this setting to further aid in patient selection. This has been facilitated by the increasing implementation of molecular pathology laboratories with comprehensive next generation sequencing (NGS) technologies. However, the significant overall costs and expertise required for the interpretation of NGS data has limited TMB evaluation in routine diagnostics, so far. This review focuses on the current use of TMB analysis in the clinical setting in the context of immune checkpoint inhibitor therapies.
Keywords: Immunotherapy; Next generation sequencing; Target therapy; Tumor mutation burden.
Conflict of interest statement
Competing interestsJutta Deckert and Raffaele Baffa are employees of Servier Pharmaceuticals. The other authors have no competing interests to declare. All the authors declare no competing interests regarding the publication of this article.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials