Efficient RT-QuIC seeding activity for α-synuclein in olfactory mucosa samples of patients with Parkinson's disease and multiple system atrophy
- PMID: 31406572
- PMCID: PMC6686411
- DOI: 10.1186/s40035-019-0164-x
Efficient RT-QuIC seeding activity for α-synuclein in olfactory mucosa samples of patients with Parkinson's disease and multiple system atrophy
Abstract
Background: Parkinson's disease (PD) is a neurodegenerative disorder whose diagnosis is often challenging because symptoms may overlap with neurodegenerative parkinsonisms. PD is characterized by intraneuronal accumulation of abnormal α-synuclein in brainstem while neurodegenerative parkinsonisms might be associated with accumulation of either α-synuclein, as in the case of Multiple System Atrophy (MSA) or tau, as in the case of Corticobasal Degeneration (CBD) and Progressive Supranuclear Palsy (PSP), in other disease-specific brain regions. Definite diagnosis of all these diseases can be formulated only neuropathologically by detection and localization of α-synuclein or tau aggregates in the brain. Compelling evidence suggests that trace-amount of these proteins can appear in peripheral tissues, including receptor neurons of the olfactory mucosa (OM).
Methods: We have set and standardized the experimental conditions to extend the ultrasensitive Real Time Quaking Induced Conversion (RT-QuIC) assay for OM analysis. In particular, by using human recombinant α-synuclein as substrate of reaction, we have assessed the ability of OM collected from patients with clinical diagnoses of PD and MSA to induce α-synuclein aggregation, and compared their seeding ability to that of OM samples collected from patients with clinical diagnoses of CBD and PSP.
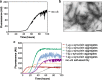
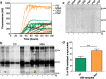
Results: Our results showed that a significant percentage of MSA and PD samples induced α-synuclein aggregation with high efficiency, but also few samples of patients with the clinical diagnosis of CBD and PSP caused the same effect. Notably, the final RT-QuIC aggregates obtained from MSA and PD samples owned peculiar biochemical and morphological features potentially enabling their discrimination.
Conclusions: Our study provide the proof-of-concept that olfactory mucosa samples collected from patients with PD and MSA possess important seeding activities for α-synuclein. Additional studies are required for (i) estimating sensitivity and specificity of the technique and for (ii) evaluating its application for the diagnosis of PD and neurodegenerative parkinsonisms. RT-QuIC analyses of OM and cerebrospinal fluid (CSF) can be combined with the aim of increasing the overall diagnostic accuracy of these diseases, especially in the early stages.
Keywords: Neurodegenerative parkinsonisms; Olfactory mucosa; Parkinson’s disease; RT-QuIC; α-Synuclein.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures


References
-
- Bhidayasiri R, Rattanachaisit W, Phokaewvarangkul O, Lim TT, Fernandez HH. Exploring bedside clinical features of parkinsonism: a focus on differential diagnosis. Parkinsonism Relat Disord. 2018. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

