Furin-mediated protein processing in infectious diseases and cancer
- PMID: 31406574
- PMCID: PMC6682551
- DOI: 10.1002/cti2.1073
Furin-mediated protein processing in infectious diseases and cancer
Abstract
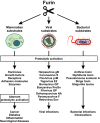
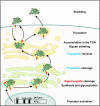
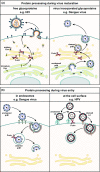
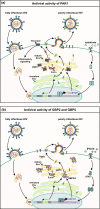
Proteolytic cleavage regulates numerous processes in health and disease. One key player is the ubiquitously expressed serine protease furin, which cleaves a plethora of proteins at polybasic recognition motifs. Mammalian substrates of furin include cytokines, hormones, growth factors and receptors. Thus, it is not surprising that aberrant furin activity is associated with a variety of disorders including cancer. Furthermore, the enzymatic activity of furin is exploited by numerous viral and bacterial pathogens, thereby enhancing their virulence and spread. In this review, we describe the physiological and pathophysiological substrates of furin and discuss how dysregulation of a simple proteolytic cleavage event may promote infectious diseases and cancer. One major focus is the role of furin in viral glycoprotein maturation and pathogenicity. We also outline cellular mechanisms regulating the expression and activation of furin and summarise current approaches that target this protease for therapeutic intervention.
Keywords: bacterial toxins; cancer; furin; guanylate‐binding proteins; proprotein convertases; viral glycoproteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Chakraborti S, Dhalla NS. Pathophysiological Aspects of Proteases. Berlin, Germany: Springer, 2017.
-
- Steiner DF, Cunningham D, Spigelman L et al Insulin biosynthesis: evidence for a precursor. Science 1967; 157: 697–700. - PubMed
-
- van de Ven WJ, Voorberg J, Fontijn R et al Furin is a subtilisin‐like proprotein processing enzyme in higher eukaryotes. Mol Biol Rep 1990; 14: 265–275. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
