A meningococcal B vaccine induces cross-protection against gonorrhea
- PMID: 31406692
- PMCID: PMC6689502
- DOI: 10.7774/cevr.2019.8.2.110
A meningococcal B vaccine induces cross-protection against gonorrhea
Abstract
Purpose: Neisseria meningitidis and Neisseria gonorrhoeae share between 80% and 90% of their genetic sequence. Meningococcal serogroup B vaccines based on outer membrane vesicles-such as VA-MENGOC-BC-could cross-protect against gonorrhea. The aim of this study was to analyze the incidence rates of gonorrhea and other sexually transmitted diseases with respect to the use of the VA-MENGOC-BC vaccine.
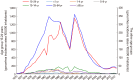
Materials and methods: Health statistics between 1970 and 2017 were reviewed and the incidence of meningococcal disease and sexually transmitted diseases (gonorrhea, syphilis, condyloma acuminatum, hepatitis B and human immunodeficiency virus infection) were analyzed during the pre- and post-vaccination periods. Gonorrhea incidence was also analyzed by age groups.
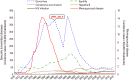
Results: VA-MENGOC-BC was successfully used to control a meningococcal epidemic in Cuba. The strategy to combat the epidemic was carried out in two stages. The first one was a nationwide mass-vaccination campaign from 1989 to 1990, targeting the population at highest-risk aged 3 months to 24 years. During the second stage, begun in 1991, it was included in the Expanded Immunization Program. Gonorrhea incidence increased from 1970 to 1989. However, after the VA-MENGOC-BC massive vaccination campaign a sharp decrease of gonorrhea incidence was observed. It lasted between 1989 and 1993. A second incidence peak was detected in 1995, but it dropped again. Data clearly show a decline in the incidence of gonorrhea following massive vaccination, in contrast with other sexually transmitted diseases. Incidence rates in unvaccinated age groups also decreased, probably due to herd immunity.
Conclusion: There is evidence that VA-MENGOC-BC could induce a moderate protection against gonorrhea.
Keywords: Gonorrhea; Neisseria gonorrhoeae; Neisseria meningitidis; Vaccines.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Abbasi J. New hope for a gonorrhea vaccine. JAMA. 2017;318:894–895. - PubMed
-
- Petousis-Harris H, Paynter J, Morgan J, et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet. 2017;390:1603–1610. - PubMed
-
- Seib KL. Gonorrhoea vaccines: a step in the right direction. Lancet. 2017;390:1567–1569. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

