Identification and Characterization of Tumor-Initiating Cells in Multiple Myeloma
- PMID: 31406992
- PMCID: PMC7225664
- DOI: 10.1093/jnci/djz159
Identification and Characterization of Tumor-Initiating Cells in Multiple Myeloma
Abstract
Background: Treatment failures in cancers, including multiple myeloma (MM), are most likely due to the persistence of a minor population of tumor-initiating cells (TICs), which are noncycling or slowly cycling and very drug resistant.
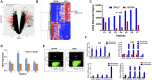
Methods: Gene expression profiling and real-time quantitative reverse transcription polymerase chain reaction were employed to define genes differentially expressed between the side-population cells, which contain the TICs, and the main population of MM cells derived from 11 MM patient samples. Self-renewal potential was analyzed by clonogenicity and drug resistance of CD24+ MM cells. Flow cytometry (n = 60) and immunofluorescence (n = 66) were applied on MM patient samples to determine CD24 expression. Therapeutic effects of CD24 antibodies were tested in xenograft MM mouse models containing three to six mice per group.
Results: CD24 was highly expressed in the side-population cells, and CD24+ MM cells exhibited high expression of induced pluripotent or embryonic stem cell genes. CD24+ MM cells showed increased clonogenicity, drug resistance, and tumorigenicity. Only 10 CD24+ MM cells were required to develop plasmacytomas in mice (n = three of five mice after 27 days). The frequency of CD24+ MM cells was highly variable in primary MM samples, but the average of CD24+ MM cells was 8.3% after chemotherapy and in complete-remission MM samples with persistent minimal residual disease compared with 1.0% CD24+ MM cells in newly diagnosed MM samples (n = 26). MM patients with a high initial percentage of CD24+ MM cells had inferior progression-free survival (hazard ratio [HR] = 3.81, 95% confidence interval [CI] = 5.66 to 18.34, P < .001) and overall survival (HR = 3.87, 95% CI = 16.61 to 34.39, P = .002). A CD24 antibody inhibited MM cell growth and prevented tumor progression in vivo.
Conclusion: Our studies demonstrate that CD24+ MM cells maintain the TIC features of self-renewal and drug resistance and provide a target for myeloma therapy.
© The Author(s) 2019. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures




References
-
- Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. - PubMed
-
- Bonnet D, Dick JE.. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. - PubMed
-
- Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. - PubMed
-
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

