Importance of time-ordered non-uniform sampling of multi-dimensional NMR spectra of Aβ1-42 peptide under aggregating conditions
- PMID: 31407200
- PMCID: PMC6819256
- DOI: 10.1007/s10858-019-00235-7
Importance of time-ordered non-uniform sampling of multi-dimensional NMR spectra of Aβ1-42 peptide under aggregating conditions
Abstract
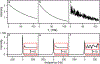
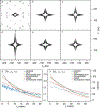
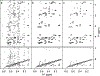
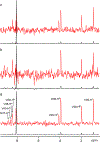
Although the order of the time steps in which the non-uniform sampling (NUS) schedule is implemented when acquiring multi-dimensional NMR spectra is of limited importance when sample conditions remain unchanged over the course of the experiment, it is shown to have major impact when samples are unstable. In the latter case, time-ordering of the NUS data points by the normalized radial length yields a reduction of sampling artifacts, regardless of the spectral reconstruction algorithm. The disadvantage of time-ordered NUS sampling is that halting the experiment prior to its completion will result in lower spectral resolution, rather than a sparser data matrix. Alternatively, digitally correcting for sample decay prior to reconstruction of randomly ordered NUS data points can mitigate reconstruction artifacts, at the cost of somewhat lower sensitivity. Application of these sampling schemes to the Alzheimer's amyloid beta (Aβ1-42) peptide at an elevated concentration, low temperature, and 3 kbar of pressure, where approximately 75% of the peptide reverts to an NMR-invisible state during the collection of a 3D 15N-separated NOESY spectrum, highlights the improvement in artifact suppression and reveals weak medium-range NOE contacts in several regions, including the C-terminal region of the peptide.
Keywords: Aggregation; High pressure; SMILE; Sampling scheme; Sparse sampling; Time ordering.
Figures


References
-
- Barna JCJ, Laue ED, Mayger MR, Skilling J & Worrall SJP Exponential Sampling, an Alternative Method for Sampling in Two-Dimensional Nmr Experiments. J. Magn. Reson 73, 69–77 (1987).
-
- Rovnyak D et al. Accelerated acquisition of high resolution triple-resonance spectra using non-uniform sampling and maximum entropy reconstruction. J. Magn. Reson 170, 15–21 (2004). - PubMed
-
- Bermel W et al. Speeding up sequence specific assignment of IDPs. J. Biomol. NMR 53, 293–301 (2012). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

