Tildrakizumab efficacy and impact on quality of life up to 52 weeks in patients with moderate-to-severe psoriasis: a pooled analysis of two randomized controlled trials
- PMID: 31407394
- PMCID: PMC6899626
- DOI: 10.1111/jdv.15862
Tildrakizumab efficacy and impact on quality of life up to 52 weeks in patients with moderate-to-severe psoriasis: a pooled analysis of two randomized controlled trials
Abstract
Background: Two randomized controlled trials (reSURFACE 1 and 2) have demonstrated the effectiveness of tildrakizumab, a high-affinity, humanized, IgG1κ, anti-interleukin-23 monoclonal antibody, for treating moderate-to-severe plaque psoriasis in the first 28 weeks.
Objectives: To examine the efficacy of tildrakizumab and its impact on quality of life (QoL) in patients with different levels of week-28 Psoriasis Area and Severity Index (PASI) improvement.
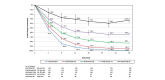
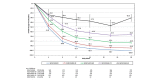
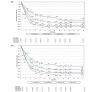
Methods: Patients treated with tildrakizumab 100 mg or 200 mg from baseline to week 28 were pooled from reSURFACE 1 and reSURFACE 2 and classified into five mutually exclusive week-28 PASI improvement groups for each dose: PASI 0-49, 50-74, 75-89, 90-99 and 100. Mean PASI improvement and Dermatology Life Quality Index (DLQI) 0/1 over time were examined for each group.
Results: Of 1156 patients, 575 were in the 100-mg and 578 in the 200-mg cohorts, respectively. At week 28, 8.3%, 14.3%, 23.8%, 30.4% and 23.1% in the 100-mg and 4.0%, 18.1%, 19.6%, 29.1% and 29.3% in the 200-mg cohort achieved PASI < 50, 50-74, 75-89, 90-99 and 100, respectively. Patients with PASI < 50 at week 28 could be identified as early as week 8, and those with week-28 PASI ≥ 90 had approximately 50% PASI improvement by week 4. Among patients achieving PASI > 50 at week 28 who continued the same dose of tildrakizumab to week 52, mean PASI improvement was maintained or improved over time. Similar results were observed for both doses. Higher proportions of patients achieved DLQI 0/1 in higher week-28 PASI groups, and DLQI 0/1 was maintained or improved to week 52. However, not all patients with PASI 100 had DLQI 0/1.
Conclusion: Patients unlikely to respond to tildrakizumab could be identified by week 8, and those likely to achieve a PASI ≥ 90 response could be identified as early as week 4. Week-28 PASI improvement level correlated with QoL improvement.
© 2019 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures

References
-
- Conrad C, Gilliet M. Psoriasis: from pathogenesis to targeted therapies. Clin Rev Allergy Immunol 2018; 54: 102–113. - PubMed
-
- Mease PJ, Gladman DD, Papp KA et al Prevalence of rheumatologist‐diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol 2013; 69: 729–735. - PubMed
-
- Han C, Lofland JH, Zhao N, Schenkel B. Increased prevalence of psychiatric disorders and health care‐associated costs among patients with moderate‐to‐severe psoriasis. J Drugs Dermatol 2011; 10: 843–850. - PubMed
-
- Hsu S, Papp KA, Lebwohl MG et al Consensus guidelines for the management of plaque psoriasis. Arch Dermatol 2012; 148: 95–102. - PubMed

