Proteolytic and nonproteolytic activation mechanisms result in conformationally and functionally different forms of coagulation factor XIII A
- PMID: 31407850
- PMCID: PMC7269131
- DOI: 10.1111/febs.15040
Proteolytic and nonproteolytic activation mechanisms result in conformationally and functionally different forms of coagulation factor XIII A
Abstract
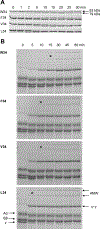
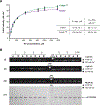
Factor XIIIA (FXIIIA) is a transglutaminase that cross-links intra- and extracellular protein substrates. FXIIIA is expressed as an inactive zymogen, and during blood coagulation, it is activated by removal of an activation peptide by the protease thrombin. No such proteolytic FXIIIA activation is known to occur in other tissues or the intracellular form of FXIIIA. For those locations, FXIIIA is assumed instead to undergo activation by Ca2+ ions. Previously, we demonstrated a monomeric state for active FXIIIA. Current analytical ultracentrifugation and kinetic experiments revealed that thrombin-activated FXIIIA has a higher conformational flexibility and a stronger affinity toward glutamine substrate than does nonproteolytically activated FXIIIA. The proteolytic activation of FXIIIA was further investigated in a context of fibrin clotting. In a series of fibrin cross-linking assays and scanning electron microscopy studies of plasma clots, the activation rates of FXIIIA V34X variants were correlated with the extent of fibrin cross-linking and incorporation of nonfibrous protein into the clot. Overall, the results suggest conformational and functional differences between active FXIIIA forms, thus expanding the understanding of FXIIIA function. Those differences may serve as a basis for developing therapeutic strategies to target FXIIIA in different physiological environments. ENZYMES: Factor XIIIA ( EC 2.3.2.13).
Keywords: analytical ultracentrifugation; factor XIII; fibrin clot; scanning electron microscopy; transglutaminase.
© 2019 Federation of European Biochemical Societies.
Conflict of interest statement
Figures


References
-
- Komaromi I, Bagoly Z & Muszbek L (2011) Factor XIII: novel structural and functional aspects, Journal of thrombosis and haemostasis : JTH. 9, 9–20. - PubMed
-
- Muszbek L, Bereczky Z, Bagoly Z, Komaromi I & Katona E (2011) Factor XIII: a coagulation factor with multiple plasmatic and cellular functions, Physiological reviews. 91, 931–72. - PubMed
-
- Schroeder V & Kohler HP (2016) Factor XIII: Structure and Function, Seminars in thrombosis and hemostasis. 42, 422–8. - PubMed
-
- Hoac B, Nelea V, Jiang W, Kaartinen MT & McKee MD (2017) Mineralization-inhibiting effects of transglutaminase-crosslinked polymeric osteopontin, Bone. 101, 37–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

