Interleukin-1β inhibits estrogen receptor-α, progesterone receptors A and B and biomarkers of human endometrial stromal cell differentiation: implications for endometriosis
- PMID: 31408162
- PMCID: PMC6821275
- DOI: 10.1093/molehr/gaz045
Interleukin-1β inhibits estrogen receptor-α, progesterone receptors A and B and biomarkers of human endometrial stromal cell differentiation: implications for endometriosis
Abstract
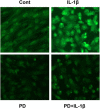
Human blastocyst nidation in the uterus and successful pregnancy require coordinated endometrial expression of estrogen receptor (ER)-α, progesterone receptors (PR)-A and -B and the gap junction protein, connexin (Cx)43. Our prior work established that inflammation associated with conditions of reduced fecundity, particularly endometriosis, can perturb eutopic decidual function. In the current studies, we have modeled endometrial decidualization in primary human endometrial stromal cell cultures derived from normal controls (NESC) and from the eutopic endometria of women with endometriosis (EESC) to test the hypothesis that a proinflammatory cytokine, interleukin (IL)-1β, can disrupt stromal cell differentiation. The cells were grown under a standard protocol with hormones (10 nM 17β-estradiol, 100 nM progesterone and 0.5 mM dibutyryl cAMP) for up to 7 days in the absence or presence of IL-1β. Time-course experiments showed that IL-1β compromised decidual function in both NESC and EESC, which was accompanied by rapid phosphorylation of ER-α, PR and Cx43 and their cellular depletion. Inhibition of the extracellular signal-regulated kinase (ERK)1/2 pathway by a selective pharmacological blocker (PD98059) or siRNA interference, or the addition of hormones themselves, blocked the phosphorylation of ERK mediators; increased the production of steroid receptors, Cx43, prolactin, insulin-like growth factor binding protein-1 (IGFBP)-1 and vascular endothelial growth factor (VEGF) and accelerated the differentiation. The results indicate that inhibition of IL-1β can enhance decidualization in NESC and EESC in vitro. Strategies to interfere with this pathway might be implemented as an in vivo approach to enhance fertility in women with endometriosis and, potentially, other inflammatory pathologies.
Keywords: connexin 43; cytokines; decidualization; endometriosis; estrogen receptor-α; inflammation; interleukin-1β; progesterone receptors; signal transduction; uterus.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For permissions, please e-mail: journals.permission@oup.com.
Figures

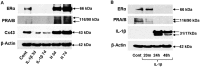
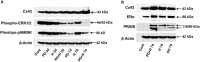




Similar articles
-
Human Endometrial Stromal Cell Differentiation is Stimulated by PPARβ/δ Activation: New Targets for Infertility?J Clin Endocrinol Metab. 2020 Sep 1;105(9):2983-95. doi: 10.1210/clinem/dgaa413. J Clin Endocrinol Metab. 2020. PMID: 32594141 Free PMC article.
-
Cabergoline Stimulates Human Endometrial Stromal Cell Decidualization and Reverses Effects of Interleukin-1β In Vitro.J Clin Endocrinol Metab. 2021 Nov 19;106(12):3591-3604. doi: 10.1210/clinem/dgab511. J Clin Endocrinol Metab. 2021. PMID: 34260712 Free PMC article.
-
Reduced connexin 43 in eutopic endometrium and cultured endometrial stromal cells from subjects with endometriosis.Mol Hum Reprod. 2014 Mar;20(3):260-70. doi: 10.1093/molehr/gat087. Epub 2013 Nov 22. Mol Hum Reprod. 2014. PMID: 24270393 Free PMC article.
-
Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer.Endocr Rev. 2013 Feb;34(1):130-62. doi: 10.1210/er.2012-1043. Epub 2013 Jan 9. Endocr Rev. 2013. PMID: 23303565 Free PMC article. Review.
-
Role of estrogen receptor-β in endometriosis.Semin Reprod Med. 2012 Jan;30(1):39-45. doi: 10.1055/s-0031-1299596. Epub 2012 Jan 23. Semin Reprod Med. 2012. PMID: 22271293 Free PMC article. Review.
Cited by
-
The Causal Effect of Urate Level on Female Infertility: A Mendelian Randomization Study.Metabolites. 2024 Sep 25;14(10):516. doi: 10.3390/metabo14100516. Metabolites. 2024. PMID: 39452897 Free PMC article.
-
Update on the pathogenesis of endometriosis-related infertility based on contemporary evidence.Front Endocrinol (Lausanne). 2025 Mar 10;16:1558271. doi: 10.3389/fendo.2025.1558271. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40130159 Free PMC article. Review.
-
Human Endometrial Stromal Cell Differentiation is Stimulated by PPARβ/δ Activation: New Targets for Infertility?J Clin Endocrinol Metab. 2020 Sep 1;105(9):2983-95. doi: 10.1210/clinem/dgaa413. J Clin Endocrinol Metab. 2020. PMID: 32594141 Free PMC article.
-
Organoid co-culture model of the human endometrium in a fully synthetic extracellular matrix enables the study of epithelial-stromal crosstalk.Med. 2023 Aug 11;4(8):554-579.e9. doi: 10.1016/j.medj.2023.07.004. Med. 2023. PMID: 37572651 Free PMC article.
-
Regulation of Inflammation Pathways and Inflammasome by Sex Steroid Hormones in Endometriosis.Front Endocrinol (Lausanne). 2020 Jan 29;10:935. doi: 10.3389/fendo.2019.00935. eCollection 2019. Front Endocrinol (Lausanne). 2020. PMID: 32063886 Free PMC article. Review.
References
-
- Arlier S, Murk W, Guzeloglu-Kayisli O, Semerci N, Larsen K, Tabak MS, Arici A, Schatz F, Lockwood CJ, Kayisli UA. The extracellular signal-regulated kinase 1/2 triggers angiogenesis in human ectopic endometrial implants by inducing angioblast differentiation and proliferation. Am J Reprod Immunol 2017;78. - PubMed
-
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007;148:3814–3826. - PubMed
-
- Dimitriadis E, Salamonsen LA, Robb L. Expression of interleukin-11 during the human menstrual cycle: coincidence with stromal cell decidualization and relationship to leukaemia inhibitory factor and prolactin. Mol Hum Reprod 2000;6:907–914. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

