Myocardial infarction triggers cardioprotective antigen-specific T helper cell responses
- PMID: 31408441
- PMCID: PMC6819128
- DOI: 10.1172/JCI123859
Myocardial infarction triggers cardioprotective antigen-specific T helper cell responses
Abstract
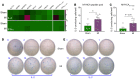
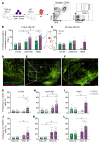
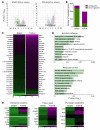
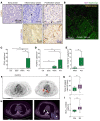
T cell autoreactivity is a hallmark of autoimmune diseases but can also benefit self-maintenance and foster tissue repair. Herein, we investigated whether heart-specific T cells exert salutary or detrimental effects in the context of myocardial infarction (MI), the leading cause of death worldwide. After screening more than 150 class-II-restricted epitopes, we found that myosin heavy chain alpha (MYHCA) was a dominant cardiac antigen triggering post-MI CD4+ T cell activation in mice. Transferred MYHCA614-629-specific CD4+ T (TCR-M) cells selectively accumulated in the myocardium and mediastinal lymph nodes (med-LN) of infarcted mice, acquired a Treg phenotype with a distinct pro-healing gene expression profile, and mediated cardioprotection. Myocardial Treg cells were also detected in autopsies from patients who suffered a MI. Noninvasive PET/CT imaging using a CXCR4 radioligand revealed enlarged med-LNs with increased cellularity in MI-patients. Notably, the med-LN alterations observed in MI patients correlated with the infarct size and cardiac function. Taken together, the results obtained in our study provide evidence showing that MI-context induces pro-healing T cell autoimmunity in mice and confirms the existence of an analogous heart/med-LN/T cell axis in MI patients.
Keywords: Adaptive immunity; Cardiology; Heart failure; Immunology; T cells.
Conflict of interest statement
Figures



Comment in
-
Regulating heart repair with cardiac-specific T lymphocytes.J Clin Invest. 2019 Nov 1;129(11):4587-4589. doi: 10.1172/JCI132441. J Clin Invest. 2019. PMID: 31609249 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

