Chemotactic migration of newly excysted juvenile Clonorchis sinensis is suppressed by neuro-antagonists
- PMID: 31408466
- PMCID: PMC6691982
- DOI: 10.1371/journal.pntd.0007573
Chemotactic migration of newly excysted juvenile Clonorchis sinensis is suppressed by neuro-antagonists
Abstract
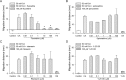
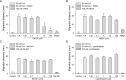
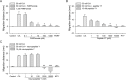
The metacercariae of the Clonorchis sinensis liver fluke excyst in the duodenum of mammalian hosts, and the newly excysted juveniles (CsNEJs) migrate along the bile duct via bile chemotaxis. Cholic acid is a major component of bile that induces this migration. We investigated the neuronal control of chemotactic behavior of CsNEJs toward cholic acid. The migration of CsNEJs was strongly inhibited at sub-micromolar concentration by dopamine D1 (LE-300 and SKF-83566), D2 (spiramide, nemonapride, and sulpiride), and D3 (GR-103691 and NGB-2904) receptor antagonists, as well as a dopamine reuptake inhibitor (BTCP). Neuropeptides, FMRFamide, peptide YY, and neuropeptide Y were also potent inhibitors of chemotaxis. Meanwhile, serotonergic, glutamatergic, and cholinergic inhibitors did not affect chemotaxis, with the exception of fluoxetine and CNQX. Confocal immunofluorescence analysis indicated that dopaminergic and cholinergic neurons were colocalized in the somatic muscle tissues of adult C. sinensis. Our findings suggest that dopaminergic neurons and neuropeptides play a major role in the chemotactic migration of CsNEJs to bile, and their inhibitors or modulators could be utilized to prevent their migration from the bile duct.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Dopaminergic antagonists inhibit bile chemotaxis of adult Clonorchis sinensis and its egg production.PLoS Negl Trop Dis. 2020 Mar 30;14(3):e0008220. doi: 10.1371/journal.pntd.0008220. eCollection 2020 Mar. PLoS Negl Trop Dis. 2020. PMID: 32226018 Free PMC article.
-
Bile acids drive chemotaxis of Clonorchis sinensis juveniles to the bile duct.PLoS Negl Trop Dis. 2018 Oct 1;12(10):e0006818. doi: 10.1371/journal.pntd.0006818. eCollection 2018 Oct. PLoS Negl Trop Dis. 2018. PMID: 30273341 Free PMC article.
-
Tracing of the Bile-chemotactic migration of juvenile Clonorchis sinensis in rabbits by PET-CT.PLoS Negl Trop Dis. 2011 Dec;5(12):e1414. doi: 10.1371/journal.pntd.0001414. Epub 2011 Dec 13. PLoS Negl Trop Dis. 2011. PMID: 22180795 Free PMC article.
-
The role of evolutionary biology in research and control of liver flukes in Southeast Asia.Infect Genet Evol. 2016 Sep;43:381-97. doi: 10.1016/j.meegid.2016.05.019. Epub 2016 May 16. Infect Genet Evol. 2016. PMID: 27197053 Free PMC article. Review.
-
Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: a brief review.BMB Rep. 2016 Nov;49(11):590-597. doi: 10.5483/bmbrep.2016.49.11.109. BMB Rep. 2016. PMID: 27418285 Free PMC article. Review.
Cited by
-
Form and Function in the Digenea, with an Emphasis on Host-Parasite and Parasite-Bacteria Interactions.Adv Exp Med Biol. 2024;1454:3-45. doi: 10.1007/978-3-031-60121-7_1. Adv Exp Med Biol. 2024. PMID: 39008262 Review.
-
Serodiagnostic antigens of Clonorchis sinensis identified and evaluated by high-throughput proteogenomics.PLoS Negl Trop Dis. 2020 Dec 28;14(12):e0008998. doi: 10.1371/journal.pntd.0008998. eCollection 2020 Dec. PLoS Negl Trop Dis. 2020. PMID: 33370333 Free PMC article.
-
Dopaminergic antagonists inhibit bile chemotaxis of adult Clonorchis sinensis and its egg production.PLoS Negl Trop Dis. 2020 Mar 30;14(3):e0008220. doi: 10.1371/journal.pntd.0008220. eCollection 2020 Mar. PLoS Negl Trop Dis. 2020. PMID: 32226018 Free PMC article.
-
Structure-based virtual screening and molecular dynamics of potential inhibitors targeting sodium-bile acid co-transporter of carcinogenic liver fluke Clonorchis sinensis.PLoS Negl Trop Dis. 2022 Nov 9;16(11):e0010909. doi: 10.1371/journal.pntd.0010909. eCollection 2022 Nov. PLoS Negl Trop Dis. 2022. PMID: 36350897 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

