Improving the evidence for indicator condition guided HIV testing in Europe: Results from the HIDES II Study - 2012 - 2015
- PMID: 31408476
- PMCID: PMC6692030
- DOI: 10.1371/journal.pone.0220108
Improving the evidence for indicator condition guided HIV testing in Europe: Results from the HIDES II Study - 2012 - 2015
Abstract
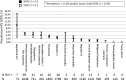
Background: It is cost-effective to perform an HIV test in people with specific indicator conditions (IC) with an undiagnosed HIV prevalence of at least 0.1%. Our aim was to determine the HIV prevalence for 14 different conditions across 20 European countries.
Methods: Individuals aged 18-65 years presenting for care with one of 14 ICs between January 2012 and June 2014 were included and routinely offered an HIV test. Logistic regression assessed factors associated with testing HIV positive. Patients presenting with infectious mononucleosis-like syndrome (IMS) were recruited up until September 2015.
Results: Of 10,877 patients presenting with an IC and included in the analysis, 303 tested positive (2.8%; 95% CI 2.5-3.1%). People presenting with an IC in Southern and Eastern Europe were more likely to test HIV positive as were people presenting with IMS, lymphadenopathy and leukocytopenia/ thrombocytopenia. One third of people diagnosed with HIV after presenting with IMS reported a negative HIV test in the preceding 12 months. Of patients newly diagnosed with HIV where data was available, 92.6% were promptly linked to care; of these 10.4% were reported lost to follow up or dead 12 months after diagnosis.
Conclusion: The study showed that 10 conditions had HIV prevalences > 0.1%. These 10 ICs should be adopted into HIV testing and IC specialty guidelines. As IMS presentation can mimic acute HIV sero-conversion and has the highest positivity rate, this IC in particular affords opportunities for earlier diagnosis and public health benefit.
Conflict of interest statement
The HIDES study was funded by the HIV in Europe initiative which has received unrestricted funding from Gilead Sciences, ViiV Healthcare, Merck, Tibotec, Pfizer, Schering-Plough, Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline. This does not alter our adherence to PLOS ONE policies on sharing data and materials. AM has received honoraria and consultancy fee from Gilead Sciences and ViiV Healthcare. JG has received honoraria and grants from ViiV Healthcare, MSD, Gilead Sciences and Janssen and is by 1 May 2018 employed by ViiV Healthcare. JR has received honoraria and consultancy fee from ViiV Healthcare, Merck/MSD, Gilead Sciences, Abbott, Abbvie, Abivax, Janssen. YY has received speaker and consultancy fee from Abbott, BMS, Gilead Sciences, MSD, Roche, Tibotec and ViiV Healthcare. KC has received consultancy and speaker fee from Gilead. All other authors declare no conflict of interest.
Figures
References
-
- HIV Indicator Conditions: Guidance for Implementing HIV Testing in Adults in Health Care Settings. Copenhagen, Copenhagen University, 2012. Available from: http://hiveurope.eu/Portals/0/Documents/Guidance.pdf.pdf?ver=2014-01-29-...
-
- Gazzard B, Clumeck N, Monforte AD, Lundgren DL. Indicator disease‐guided testing for HIV–the next step for Europe? HIV Medicine. 2008. July;9(s2):34–40. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials