Energetic substrate availability regulates synchronous activity in an excitatory neural network
- PMID: 31408504
- PMCID: PMC6692003
- DOI: 10.1371/journal.pone.0220937
Energetic substrate availability regulates synchronous activity in an excitatory neural network
Abstract
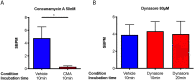
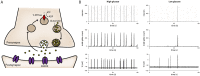
Neural networks are required to meet significant metabolic demands associated with performing sophisticated computational tasks in the brain. The necessity for efficient transmission of information imposes stringent constraints on the metabolic pathways that can be used for energy generation at the synapse, and thus low availability of energetic substrates can reduce the efficacy of synaptic function. Here we study the effects of energetic substrate availability on global neural network behavior and find that glucose alone can sustain excitatory neurotransmission required to generate high-frequency synchronous bursting that emerges in culture. In contrast, obligatory oxidative energetic substrates such as lactate and pyruvate are unable to substitute for glucose, indicating that processes involving glucose metabolism form the primary energy-generating pathways supporting coordinated network activity. Our experimental results are discussed in the context of the role that metabolism plays in supporting the performance of individual synapses, including the relative contributions from postsynaptic responses, astrocytes, and presynaptic vesicle cycling. We propose a simple computational model for our excitatory cultures that accurately captures the inability of metabolically compromised synapses to sustain synchronous bursting when extracellular glucose is depleted.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Lactate as a supplemental fuel for synaptic transmission and neuronal network oscillations: Potentials and limitations.J Neurochem. 2024 May;168(5):608-631. doi: 10.1111/jnc.15867. Epub 2023 Jun 13. J Neurochem. 2024. PMID: 37309602 Review.
-
Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging.Philos Trans R Soc Lond B Biol Sci. 1999 Jul 29;354(1387):1155-63. doi: 10.1098/rstb.1999.0471. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10466143 Free PMC article. Review.
-
Modeling of substance P and 5-HT induced synaptic plasticity in the lamprey spinal CPG: consequences for network pattern generation.J Comput Neurosci. 2001 Sep-Oct;11(2):183-200. doi: 10.1023/a:1012806018730. J Comput Neurosci. 2001. PMID: 11717534
-
A New Computational Model for Astrocytes and Their Role in Biologically Realistic Neural Networks.Comput Intell Neurosci. 2018 Jul 5;2018:3689487. doi: 10.1155/2018/3689487. eCollection 2018. Comput Intell Neurosci. 2018. PMID: 30073021 Free PMC article.
-
Synaptic energy drives the information processing mechanisms in spiking neural networks.Math Biosci Eng. 2014 Apr;11(2):233-56. Math Biosci Eng. 2014. PMID: 24245716
Cited by
-
Electrophysiological In Vitro Study of Long-Range Signal Transmission by Astrocytic Networks.Adv Sci (Weinh). 2023 Oct;10(29):e2301756. doi: 10.1002/advs.202301756. Epub 2023 Jul 23. Adv Sci (Weinh). 2023. PMID: 37485646 Free PMC article.
-
Glucose-Sparing Action of Ketones Boosts Functions Exclusive to Glucose in the Brain.eNeuro. 2020 Nov 9;7(6):ENEURO.0303-20.2020. doi: 10.1523/ENEURO.0303-20.2020. Print 2020 Nov-Dec. eNeuro. 2020. PMID: 33168619 Free PMC article.
-
Brain energy failure in dementia syndromes: Opportunities and challenges for glucagon-like peptide-1 receptor agonists.Alzheimers Dement. 2022 Mar;18(3):478-497. doi: 10.1002/alz.12474. Epub 2021 Oct 14. Alzheimers Dement. 2022. PMID: 34647685 Free PMC article. Review.
-
The Role of Ketogenic Metabolic Therapy on the Brain in Serious Mental Illness: A Review.J Psychiatr Brain Sci. 2022;7(5):e220009. doi: 10.20900/jpbs.20220009. Epub 2022 Oct 31. J Psychiatr Brain Sci. 2022. PMID: 36483840 Free PMC article.
-
Schizophrenia: a disorder of broken brain bioenergetics.Mol Psychiatry. 2022 May;27(5):2393-2404. doi: 10.1038/s41380-022-01494-x. Epub 2022 Mar 9. Mol Psychiatry. 2022. PMID: 35264726 Review.
References
-
- Ames A 3rd. CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000;34: 42–68. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

