Development and evaluation of novel bio-safe filter paper-based kits for sputum microscopy and transport to directly detect Mycobacterium tuberculosis and associated drug resistance
- PMID: 31408508
- PMCID: PMC6692035
- DOI: 10.1371/journal.pone.0220967
Development and evaluation of novel bio-safe filter paper-based kits for sputum microscopy and transport to directly detect Mycobacterium tuberculosis and associated drug resistance
Abstract
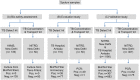
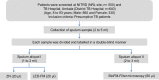
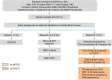
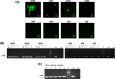
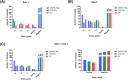
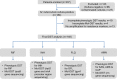
India has the highest burden of Tuberculosis (TB) and multidrug-resistant TB (MDR-TB) worldwide. Innovative technology is the need of the hour to identify these cases that remain either undiagnosed or inadequately diagnosed due to the unavailability of appropriate tools at primary healthcare settings. We developed and evaluated 3 kits, namely 'TB Detect' (containing BioFM-Filter device), 'TB Concentration and Transport' (containing Trans-Filter device) and 'TB DNA Extraction' kits. These kits enable bio-safe equipment-free concentration of sputum on filters and improved fluorescence microscopy at primary healthcare centres, ambient temperature transport of dried inactivated sputum filters to central laboratories and molecular detection of drug resistance by PCR and DNA sequencing (Mol-DST). In a 2-site evaluation (n = 1190 sputum specimens) on presumptive TB patients, BioFM-Filter smear exhibited a significant increase in positivity of 7% and 4% over ZN smear and LED-FM smear (p<0.05), respectively and an increment in smear grade status (1+ or 2+ to 3+) of 16% over ZN smear and 20% over LED-FM smear. The sensitivity of Mol-DST in presumptive MDR-TB and XDR-TB cases (n = 148) was 90% for Rifampicin (95% confidence interval [CI], 78-96%), 84% for Isoniazid (95% CI, 72-92%), 83% for Fluoroquinolones (95% CI, 66-93%) and 75% for Aminoglycosides (95% CI, 35-97%), using phenotypic DST as the reference standard. Test specificity was 88-93% and concordance was ~89-92% (κ value 0.8-0.9). The patient-friendly kits described here address several of the existing challenges and are designed to provide 'Universal Access' to rapid TB diagnosis, including drug-resistant disease. Their utility was demonstrated by application to sputum at 2 sites in India. Our findings pave the way for larger studies in different point-of-care settings, including high-density urban areas and remote geographical locations.
Conflict of interest statement
The authors have read the journal’s policy and the authors of this manuscript state the following competing interests: NKG and AG are Managing and Executive directors of the company ‘Advanced Microdevices Pvt. Ltd.’ (mdi) respectively, the partial funder of this study. All the other authors are joint inventors with mdi in an Indian Provisional Patent application named ‘Apparatus and method for processing a sample for rapid diagnosis of tuberculosis and safe transport of bacteria’, (Patent application number- 201811042155). This does not alter the authors’ adherence to all the ‘PLOS ONE’ policies on sharing data and materials, as detailed online in the guide for authors.
Figures


References
-
- WHO. Global Tuberculosis Report. 2018.
-
- WHO. Fluorescent light-emitting diode (LED) microscopy for diagnosis of tuberculosis: Policy Statement. 2011. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

