Prioritization of candidate cancer drugs based on a drug functional similarity network constructed by integrating pathway activities and drug activities
- PMID: 31408580
- PMCID: PMC6763777
- DOI: 10.1002/1878-0261.12564
Prioritization of candidate cancer drugs based on a drug functional similarity network constructed by integrating pathway activities and drug activities
Abstract
Due to the speed, efficiency, relative risk, and lower costs compared to traditional drug discovery, the prioritization of candidate drugs for repurposing against cancers of interest has attracted the attention of experts in recent years. Herein, we present a powerful computational approach, termed prioritization of candidate drugs (PriorCD), for the prioritization of candidate cancer drugs based on a global network propagation algorithm and a drug-drug functional similarity network constructed by integrating pathway activity profiles and drug activity profiles. This provides a new approach to drug repurposing by first considering the drug functional similarities at the pathway level. The performance of PriorCD in drug repurposing was evaluated by using drug datasets of breast cancer and ovarian cancer. Cross-validation tests on the drugs approved for the treatment of these cancers indicated that our approach can achieve area under receiver-operating characteristic curve (AUROC) values greater than 0.82. Furthermore, literature searches validated our results, and comparison with other classical gene-based repurposing methods indicated that our pathway-level PriorCD is comparatively more effective at prioritizing candidate drugs with similar therapeutic effects. We hope that our study will be of benefit to the field of drug discovery. In order to expand the usage of PriorCD, a freely available R-based package, PriorCD, has been developed to prioritize candidate anticancer drugs for drug repurposing.
Keywords: drug activities; drug functional similarity network; drug repurposing; pathway activities.
© 2019 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

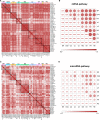


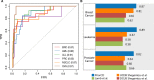
References
-
- Ashburn TT and Thor KB (2004) Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discovery 3, 673. - PubMed
-
- Beggiolin G (2005) Preclinical antitumor activity of CT‐2106 (polyglutamate camptothecin) in human ovarian carcinoma xenograft. Can Res 65, 329–330.
-
- Benjamini Y and Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57, 289–300.
-
- Blower PE, Verducci JS, Lin S, Zhou J, Chung JH, Dai Z, Liu CG, Reinhold W, Lorenzi PL, Kaldjian EP et al (2007) MicroRNA expression profiles for the NCI‐60 cancer cell panel. Mol Cancer Ther 6, 1483–1491. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

