CD82 controls CpG-dependent TLR9 signaling
- PMID: 31408613
- PMCID: PMC6988855
- DOI: 10.1096/fj.201901547R
CD82 controls CpG-dependent TLR9 signaling
Abstract
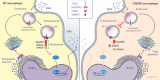
The tetraspanin CD82 is a potent suppressor of tumor metastasis and regulates several processes including signal transduction, cell adhesion, motility, and aggregation. However, the mechanisms by which CD82 participates in innate immunity are unknown. We report that CD82 is a key regulator of TLR9 trafficking and signaling. TLR9 recognizes unmethylated cytosine-phosphate-guanine (CpG) motifs present in viral, bacterial, and fungal DNA. We demonstrate that TLR9 and CD82 associate in macrophages, which occurs in the endoplasmic reticulum (ER) and post-ER. Moreover, CD82 is essential for TLR9-dependent myddosome formation in response to CpG stimulation. Finally, CD82 modulates TLR9-dependent NF-κB nuclear translocation, which is critical for inflammatory cytokine production. To our knowledge, this is the first time a tetraspanin has been implicated as a key regulator of TLR signaling. Collectively, our study demonstrates that CD82 is a specific regulator of TLR9 signaling, which may be critical in cancer immunotherapy approaches and coordinating the innate immune response to pathogens.-Khan, N. S., Lukason, D. P., Feliu, M., Ward, R. A., Lord, A. K., Reedy, J. L., Ramirez-Ortiz, Z. G., Tam, J. M., Kasperkovitz, P. V., Negoro, P. E., Vyas, T. D., Xu, S., Brinkmann, M. M., Acharaya, M., Artavanis-Tsakonas, K., Frickel, E.-M., Becker, C. E., Dagher, Z., Kim, Y.-M., Latz, E., Ploegh, H. L., Mansour, M. K., Miranti, C. K., Levitz, S. M., Vyas, J. M. CD82 controls CpG-dependent TLR9 signaling.
Keywords: TLRs; macrophages; myddosome; tetraspanins.
Conflict of interest statement
The authors thank Nicole Wolf for the artwork displayed in the graphical abstract (Fig. 7), Shizuo Akira (Osaka University, Osaka, Japan) for the TLR9 knockout (TLR9KO) mice, and Douglas Golenbock (University of Massachusetts Medical Center, Worcester, MA, USA) for the TLR9KO macrophage cell line. The authors also thank Kensuyke Miyake and Ryutaro Fukui (University of Tokyo, Tokyo, Japan) for the TLR9 monoclonal and polyclonal antibodies (41), and Gregory Barton and Bo Liu (University of California–Berkeley, Berkeley, CA, USA) for the TLR7-FLAG construct. This work was supported by U.S. National Institutes of Health, National Institute of Allergy and Infectious Diseases Grants R01 AI092084 and R01 AI097519 (to J.M.V.) and R01 AI025780 and R01 AI139615 (to S.M.L.). This work was also supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001076), the UK Medical Research Council (FC001076), and the Wellcome Trust (FC001076). The authors declare no conflicts of interest.
Figures






Similar articles
-
Size-dependent attenuation of TLR9 signaling by gold nanoparticles in macrophages.J Immunol. 2012 Jan 1;188(1):68-76. doi: 10.4049/jimmunol.1100344. Epub 2011 Dec 7. J Immunol. 2012. PMID: 22156340
-
CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion.J Cell Biol. 2006 Mar 27;172(7):1057-68. doi: 10.1083/jcb.200508058. J Cell Biol. 2006. PMID: 16567503 Free PMC article.
-
Tetraspanin CD82 Organizes Dectin-1 into Signaling Domains to Mediate Cellular Responses to Candida albicans.J Immunol. 2019 Jun 1;202(11):3256-3266. doi: 10.4049/jimmunol.1801384. Epub 2019 Apr 22. J Immunol. 2019. PMID: 31010852 Free PMC article.
-
[Multilevel maturation of Toll-like receptor 9].Postepy Hig Med Dosw (Online). 2013 Oct 30;67:1034-46. doi: 10.5604/17322693.1074013. Postepy Hig Med Dosw (Online). 2013. PMID: 24184955 Review. Polish.
-
Immunotherapeutic potential of CpG oligodeoxynucleotides in veterinary species.Immunopharmacol Immunotoxicol. 2013 Oct;35(5):535-44. doi: 10.3109/08923973.2013.828743. Epub 2013 Aug 28. Immunopharmacol Immunotoxicol. 2013. PMID: 23981003 Review.
Cited by
-
Recombinant human KAI1/CD82 attenuates M1 macrophage polarization on LPS-stimulated RAW264.7 cells via blocking TLR4/JNK/NF-κB signal pathway.BMB Rep. 2023 Jun;56(6):359-364. doi: 10.5483/BMBRep.2022-0189. BMB Rep. 2023. PMID: 36945827 Free PMC article.
-
Targeting CD82/KAI1 for Precision Therapeutics in Surmounting Metastatic Potential in Breast Cancer.Cancers (Basel). 2021 Sep 6;13(17):4486. doi: 10.3390/cancers13174486. Cancers (Basel). 2021. PMID: 34503296 Free PMC article. Review.
-
Toll-like Receptor Mediation in SARS-CoV-2: A Therapeutic Approach.Int J Mol Sci. 2022 Sep 14;23(18):10716. doi: 10.3390/ijms231810716. Int J Mol Sci. 2022. PMID: 36142620 Free PMC article. Review.
-
Tetraspanins in the regulation of mast cell function.Med Microbiol Immunol. 2020 Aug;209(4):531-543. doi: 10.1007/s00430-020-00679-x. Epub 2020 Jun 7. Med Microbiol Immunol. 2020. PMID: 32507938 Free PMC article. Review.
-
Regulation of the nucleic acid-sensing Toll-like receptors.Nat Rev Immunol. 2022 Apr;22(4):224-235. doi: 10.1038/s41577-021-00577-0. Epub 2021 Jul 16. Nat Rev Immunol. 2022. PMID: 34272507 Free PMC article. Review.
References
-
- Hemler M. E. (2005) Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 6, 801–811 - PubMed
-
- Van Spriel A. B., Figdor C. G. (2010) The role of tetraspanins in the pathogenesis of infectious diseases. Microbes Infect. 12, 106–112 - PubMed
-
- Hemler M. E. (2014) Tetraspanin proteins promote multiple cancer stages. Nat. Rev. Cancer 14, 49–60 - PubMed
-
- Yáñez-Mó M., Barreiro O., Gordon-Alonso M., Sala-Valdés M., Sánchez-Madrid F. (2009) Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 19, 434–446 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

